Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

R is a constant for the particular gas whose molecular weight is M.
Alternative units for R are kJ/kg
?
K, Btu/lb
?
8R, and ft
?
lbf/lb
?
8R.
Table 3.1 provides a sampling of values for the gas constant R calcu-
lated from Eq. 3.25.
Equation 3.21 can be expressed in terms of the compressibility fac-
tor as
lim
p
S0
Z 5 1 (3.26)
That is, the compressibility factor Z tends to unity as pressure tends
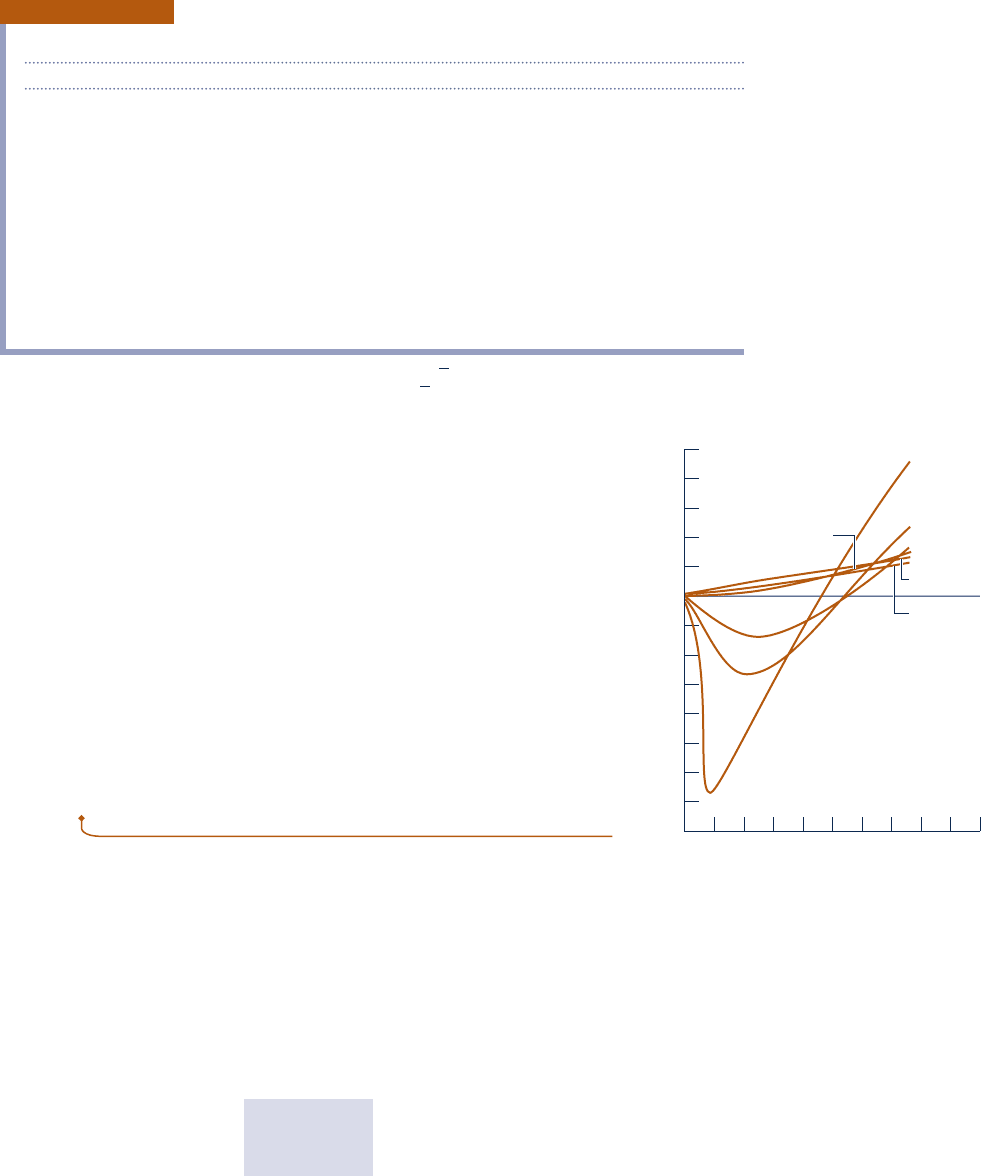
to zero at fixed temperature. This can be illustrated by reference to
Fig. 3.11, which shows Z for hydrogen plotted versus pressure at a
number of different temperatures. In general, at states of a gas where
pressure is small relative to the critical pressure, Z is approximately 1.
3.11.3
Generalized Compressibility Data, Z Chart
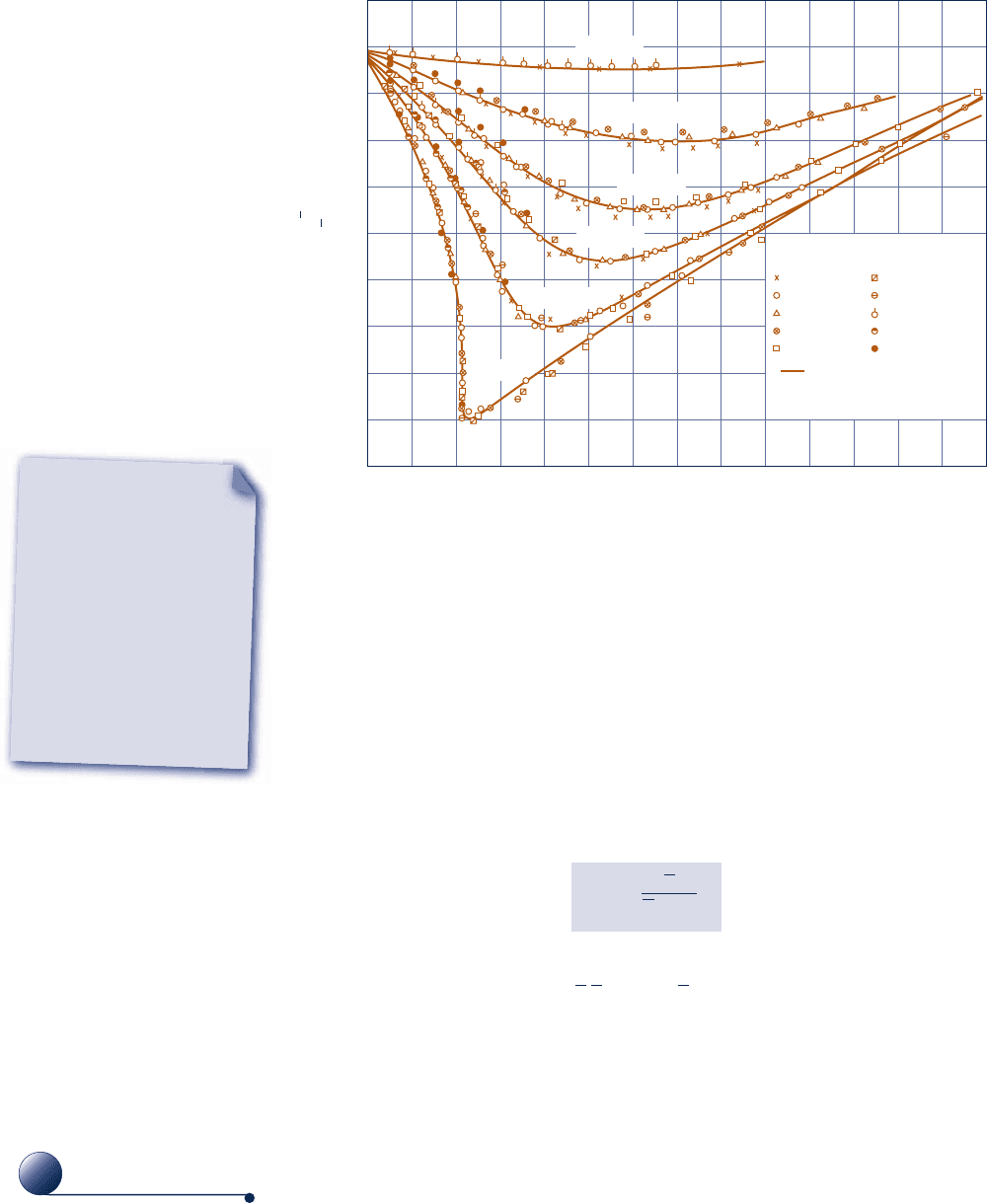
Figure 3.11 gives the compressibility factor for hydrogen versus pres-
sure at specified values of temperature. Similar charts have been pre-
pared for other gases. When these charts are studied, they are found
to be qualitatively similar. Further study shows that when the coordi-
nates are suitably modified, the curves for several different gases coin-
cide closely when plotted together on the same coordinate axes, and so quantitative
similarity also can be achieved. This is referred to as the principle of corresponding
states. In one such approach, the compressibility factor Z is plotted versus a dimen-
sionless
reduced pressure p
R
and reduced temperature T
R
, defined as
p
R
5 p
/
p
c
(3.27)
T
R
5 T
/
T
c
(3.28)
where p
c
and T
c
denote the critical pressure and temperature, respectively. This results
in a generalized compressibility chart of the form Z 5 f(p
R
, T
R
). Figure 3.12 shows
experimental data for 10 different gases on a chart of this type. The solid lines denot-
ing reduced isotherms represent the best curves fitted to the data. Observe that
Tables A-1 provide the critical temperature and critical pressure for a sampling of
substances.
1.5
1.0
0.5
0 100 200
35 K (63°R)
50 K (90°R)
60 K (108°R)
200 K (360°R)
300 K (540°R)
100 K (180°R)
Z
p (atm)
Fig. 3.11 Variation of the compressibility
factor of hydrogen with pressure at constant
temperature.
Values of the Gas Constant R of Selected Elements and Compounds
Substance Chemical Formula R (kJ/kg
?
K) R (Btu/lb
?
8R)
Air — 0.2870 0.06855
Ammonia NH
3
0.4882 0.11662
Argon Ar 0.2082 0.04972
Carbon dioxide CO
2
0.1889 0.04513
Carbon monoxide CO 0.2968 0.07090
Helium He 2.0769 0.49613
Hydrogen H
2
4.1240 0.98512
Methane CH
4
0.5183 0.12382
Nitrogen N
2
0.2968 0.07090
Oxygen O
2
0.2598 0.06206
Water H
2
O 0.4614 0.11021
TABLE 3.1
Source: R values are calculated in terms of the universal gas constant R 5 8.314 kJ
/
kmol ? K 5 1.986 Btu
/
lbmol ? 8R
and the molecular weight M provided in Table A-1 using R 5 R
/
M (Eq. 3.25).
reduced pressure
and temperature
3.11 Generalized Compressibility Chart 123
c03EvaluatingProperties.indd Page 123 5/19/10 8:32:23 PM user-s146 c03EvaluatingProperties.indd Page 123 5/19/10 8:32:23 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
124 Chapter 3 Evaluating Properties
A generalized chart more suitable for problem solving than Fig. 3.12 is given in
the Appendix as Figs. A-1, A-2, and A-3. In Fig. A-1, p
R
ranges from 0 to 1.0; in
Fig. A-2, p
R
ranges from 0 to 10.0; and in Fig. A-3, p
R
ranges from 10.0 to 40.0. At
any one temperature, the deviation of observed values from those of the general-
ized chart increases with pressure. However, for the 30 gases used in developing
the chart, the deviation is at most on the order of 5% and for most ranges is much
less.
1
Values of specific volume are included on the generalized chart through the vari-
able y9
R
, called the pseudoreduced specific volume, defined by
y¿
R
5
y
RT
c
y
p
c
(3.29)
The pseudoreduced specific volume gives a better correlation of the data than does
the reduced specific volume y
R
5 y
/
y
c
, where y
c
is the critical specific volume.
Using the critical pressure and critical temperature of a substance of interest, the
generalized chart can be entered with various pairs of the variables T
R
, p
R
, and
y 9
R
: (T
R
, p
R
), (p
R
, y9
R
), or (T
R
, y9
R
). The merit of the generalized chart for relating p,
y, and T data for gases is simplicity coupled with accuracy. However, the generalized
compressibility chart should not be used as a substitute for p–y–T data for a given
substance as provided by tables or computer software. The chart is mainly useful for
obtaining reasonable estimates in the absence of more accurate data.
The next example provides an illustration of the use of the generalized compress-
ibility chart.
1
To determine Z for hydrogen, helium, and neon above a T
R
of 5, the reduced temperature and pressure
should be calculated using T
R
5 T/(T
c
1 8) and p
R
5 p/(p
c
1 8), where temperatures are in K and pres-
sures are in atm.
pseudoreduced
specific volume
T
R
= 1.00
Z =
pv
–––
RT
=
pv
–––
RT
1.1
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0 1.0 2.0 3.0 4.0 5.0 6.0 7.00.5 1.5 2.5 3.5 4.5 5.5 6.5
Methane
Ethylene
Ethane
Propane
n-Butane
Isopentane
n-Heptane
Nitrogen
Carbon dioxide
Water
Average curve based on
data on hydrocarbons
Legend
Reduced pressure p
R
T
R
= 2.00
T
R
= 1.50
T
R
= 1.30
T
R
= 1.20
T
R
= 1.10
Fig. 3.12 Generalized compressibility chart for various gases.
generalized compressibility
chart
TAKE NOTE...
Study of Fig. A-2 shows
that the value of Z tends
to unity at fixed reduced
temperature T
R
as reduced
pressure p
R
tends to zero.
That is, Z
S
1
as p
R
S
0 at fixed T
R
.
Figure A-2 also shows that
Z tends to unity at fixed
reduced pressure as reduced
temperature becomes large.
A
A
Ideal_Gas
A.15 – Tab a
c03EvaluatingProperties.indd Page 124 6/30/10 10:54:31 AM user-s146 c03EvaluatingProperties.indd Page 124 6/30/10 10:54:31 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Using the Generalized Compressibility Chart
c c c c EXAMPLE 3.7 c
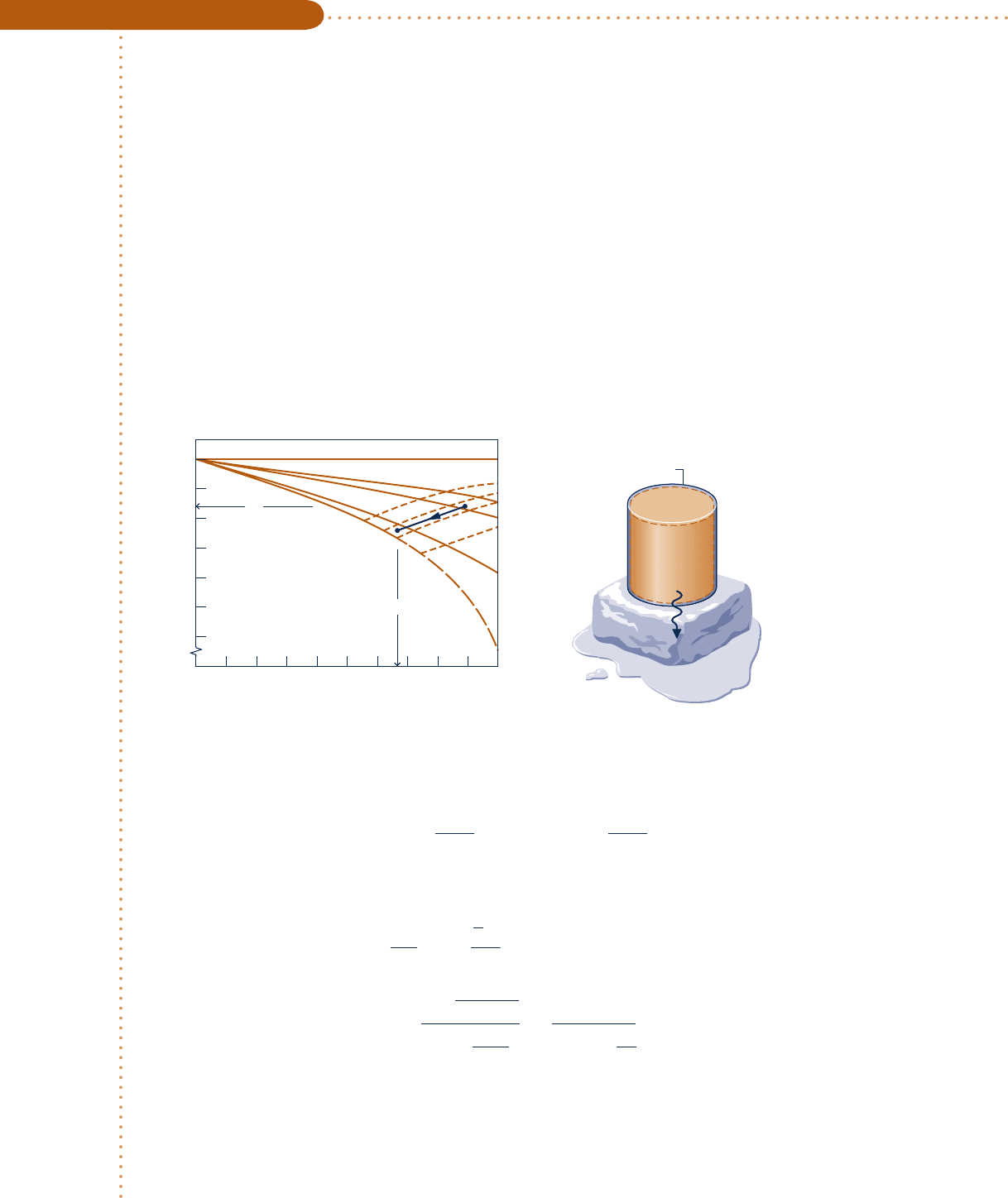
A closed, rigid tank filled with water vapor, initially at 20 MPa, 5208C, is cooled until its temperature reaches
4008C. Using the compressibility chart, determine
(a) the specific volume of the water vapor in m
3
/kg at the initial state.
(b) the pressure in MPa at the final state.
Compare the results of parts (a) and (b) with the values obtained from the superheated vapor table, Table A-4.
SOLUTION
Known:
Water vapor is cooled at constant volume from 20 MPa, 5208C to 4008C.
Find: Use the compressibility chart and the superheated vapor table to determine the specific volume and final
pressure and compare the results.
Schematic and Given Data:
Analysis:
(a) From Table A-1, T
c
5 647.3 K and p
c
5 22.09 MPa for water. Thus
➊
T
R1
5
793
6
4
7.3
5 1.23,
p
R1
5
20
22
.09
5 0.91
With these values for the reduced temperature and reduced pressure, the value of Z obtained from Fig. A-1 is
approximately 0.83. Since Z 5 py/RT, the specific volume at state 1 can be determined as follows:
y
1
5 Z
1
RT
1
p
1
5 0.83
RT
1
M
p
1
➋
5 0.83 ±
8314
N ?
m
kmol ? K
18.02
k
g
kmol
≤
°
79
3 K
20 3 10
6
N
m
2
¢
5 0.0152 m
3
/
kg
The molecular weight of water is from Table A-1.
Engineering Model:
1.
The water vapor is
a closed system.
2. The initial and
final states are at
equilibrium.
3. The volume is
constant.
Fig. E3.7
Z
1
p
R2
1.0
0.5
Z =
pv
–––
RT
0 0.5
p
R
1.0
v´
R
= 1.2
v´
R
= 1.1
T
R
= 1.3
1
2
T
R
= 1.2
T
R
= 1.05
Water
vapor
Cooling
Block of ice
Closed, rigid tank
p
1
=
T
1
=
T
2
=
20 MPa
520°C
400°C
3.11 Generalized Compressibility Chart 125
c03EvaluatingProperties.indd Page 125 5/27/10 7:33:11 AM user-f391 c03EvaluatingProperties.indd Page 125 5/27/10 7:33:11 AM user-f391 /Users/user-f391/Desktop/27MAY/Users/user-f391/Desktop/27MAY
126 Chapter 3
Evaluating Properties
3.11.4
Equations of State
Considering the curves of Figs. 3.11 and 3.12, it is reasonable to think that the varia-
tion with pressure and temperature of the compressibility factor for gases might be
expressible as an equation, at least for certain intervals of p and T. Two expressions
can be written that enjoy a theoretical basis. One gives the compressibility factor as
an infinite series expansion in pressure:
Z 5 1 1 B
ˆ
1T2p 1 C
ˆ
1T2p
2
1 D
ˆ
1T2p
3
1
. . .
(3.30)
where the coefficients B
ˆ
, C
ˆ
, D
ˆ
, . . . depend on temperature only. The dots in Eq. 3.30
represent higher-order terms. The other is a series form entirely analogous to Eq. 3.30
but expressed in terms of 1
/
y instead of p
Z 5 1 1
B1T
2
y
1
C1T
2
y
2
1
D1T
2
y
3
1
. . .
(3.31)
Equations 3.30 and 3.31 are known as
virial equations of state, and the coefficients
B
ˆ
, C
ˆ
, D
ˆ
, . . . and B, C, D, . . . are called virial coefficients. The word virial stems from
the Latin word for force. In the present usage it is force interactions among molecules
that are intended.
Turning to Table A-4, the specific volume at the initial state is 0.01551 m
3
/kg. This is in good agreement with
the compressibility chart value, as expected.
(b) Since both mass and volume remain constant, the water vapor cools at constant specific volume, and thus
at constant y9
R
. Using the value for specific volume determined in part (a), the constant y9
R
value is
y¿
R
5
yp
c
RT
c
5
a0.0152
m
3
kg
ba22.09 3 10
6
N
m
2
b
a
831
4
18.02
N ? m
kg ? K
b1647.3 K2
5 1.12
At state 2
T
R2
5
673
647.3
5 1.04
Locating the point on the compressibility chart where y9
R
5 1.12 and T
R
5 1.04, the corresponding value for p
R
is about 0.69. Accordingly
p
2
5 p
c
1p
R2
25 122.09 MPa210.6925 15.24 MPa
Interpolating in the superheated vapor tables gives p
2
5 15.16 MPa. As before,
the compressibility chart value is in good agreement with the table value.
➊ Absolute temperature and absolute pressure must be used in evaluating
the compressibility factor Z, the reduced temperature T
R
, and reduced
pressure p
R
.
➋ Since Z is unitless, values for p, y, R, and T must be used in consistent
units.
Using the compressibility chart, determine the specific vol-
ume, in m
3
/kg, for water vapor at 14 MPa, 4408C. Compare with the steam
table value. Ans. 0.0195 m
3
/kg
virial equations of state
Ability to…
❑
retrieve p–y–T data from the
generalized compressibility
chart.
❑
retrieve p–y–T data from
the steam tables..
✓
Skills Developed
c03EvaluatingProperties.indd Page 126 5/19/10 8:32:27 PM user-s146 c03EvaluatingProperties.indd Page 126 5/19/10 8:32:27 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
The virial expansions can be derived by the methods of statistical mechanics, and
physical significance can be attributed to the coefficients: B
/
y accounts for two-
molecule interactions, C
/
y
2
accounts for three-molecule interactions, etc. In principle,
the virial coefficients can be calculated by using expressions from statistical mechan-
ics derived from consideration of the force fields around the molecules of a gas. The
virial coefficients also can be determined from experimental p–y–T data. The virial
expansions are used in Sec. 11.1 as a point of departure for the further study of ana-
lytical representations of the p–y–T relationship of gases known generically as equa-
tions of state.
The virial expansions and the physical significance attributed to the terms making
up the expansions can be used to clarify the nature of gas behavior in the limit as
pressure tends to zero at fixed temperature. From Eq. 3.30 it is seen that if pressure
decreases at fixed temperature, the terms B
ˆ
p, C
ˆ
p
2
, etc. accounting for various molec-
ular interactions tend to decrease, suggesting that the force interactions become
weaker under these circumstances. In the limit as pressure approaches zero, these
terms vanish, and the equation reduces to Z 5 1 in accordance with Eq. 3.26. Simi-
larly, since specific volume increases when the pressure decreases at fixed tempera-
ture, the terms B
/
y , C
/
y
2
, etc. of Eq. 3.31 also vanish in the limit, giving Z 5 1 when
the force interactions between molecules are no longer significant.
Evaluating Properties Using
the Ideal Gas Model
3.12 Introducing the Ideal Gas Model
In this section the ideal gas model is introduced. The ideal gas model has many applica-
tions in engineering practice and is frequently used in subsequent sections of this text.
3.12.1
Ideal Gas Equation of State
As observed in Sec. 3.11.3, study of the generalized compressibility chart Fig. A-2
shows that at states where the pressure p is small relative to the critical pressure p
c
(low p
R
) and/or the temperature T is large relative to the critical temperature T
c
(high
T
R
), the compressibility factor, Z 5 py/RT, is approximately 1. At such states, we can
assume with reasonable accuracy that Z 5 1, or
py 5 RT (3.32)
Known as the ideal gas equation of state, Eq. 3.32 underlies the second part of this
chapter dealing with the ideal gas model.
Alternative forms of the same basic relationship among pressure, specific volume,
and temperature are obtained as follows. With y 5 V/m, Eq. 3.32 can be expressed as
pV 5 mRT (3.33)
In addition, using y 5 y
/
M and R 5 R
/
M, which are Eqs. 1.9 and 3.25, respectively,
where M is the molecular weight, Eq. 3.32 can be expressed as
p
y
5 RT (3.34)
or, with y 5 V
/
n, as
pV 5 nRT (3.35)
ideal gas equation
of state
3.12 Introducing the Ideal Gas Model 127
A
A
Ideal_Gas
A.15 – Tab b
c03EvaluatingProperties.indd Page 127 6/30/10 10:56:09 AM user-s146 c03EvaluatingProperties.indd Page 127 6/30/10 10:56:09 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
128 Chapter 3 Evaluating Properties
3.12.2
Ideal Gas Model
For any gas whose equation of state is given exactly by p
y
5 RT, the specific internal
energy depends on temperature only. This conclusion is demonstrated formally in Sec.
11.4. It is also supported by experimental observations, beginning with the work of
Joule, who showed in 1843 that the internal energy of air at low density (large specific
volume) depends primarily on temperature. Further motivation from the microscopic
viewpoint is provided shortly. The specific enthalpy of a gas described by p
y
5 RT
also depends on temperature only, as can be shown by combining the definition of
enthalpy, h 5 u 1 p
y
, with u 5 u(T) and the ideal gas equation of state to obtain
h 5 u(T) 1 RT. Taken together, these specifications constitute the ideal gas model,
summarized as follows
p
y 5 RT (3.32)
u 5 u1T2 (3.36)
h 5 h1T25 u1T21 RT (3.37)
The specific internal energy and enthalpy of gases generally depend on two
independent properties, not just temperature as presumed by the ideal gas model.
Moreover, the ideal gas equation of state does not provide an acceptable approx-
imation at all states. Accordingly, whether the ideal gas model is used depends on
the error acceptable in a given calculation. Still, gases often do approach ideal gas
behavior, and a particularly simplified description is obtained with the ideal gas
model.
To verify that a gas can be modeled as an ideal gas, the states of interest can be
located on a compressibility chart to determine how well Z 5 1 is satisfied. As shown
in subsequent discussions, other tabular or graphical property data can also be used
to determine the suitability of the ideal gas model.
The next example illustrates the use of the ideal gas equation of state and rein-
forces the use of property diagrams to locate principal states during processes.
ideal gas model
Analyzing Air as an Ideal Gas Undergoing a Thermodynamic Cycle
c c c c EXAMPLE 3.8 c
One pound of air in a piston–cylinder assembly undergoes a thermodynamic cycle consisting of three processes.
Process 1–2: Constant specific volume
Process 2–3: Constant-temperature expansion
Process 3–1: Constant-pressure compression
At state 1, the temperature is 5408R, and the pressure is 1 atm. At state 2, the pressure is 2 atm. Employing the
ideal gas equation of state,
(a) sketch the cycle on p–y coordinates.
(b) determine the temperature at state 2, in 8R.
(c) determine the specific volume at state 3, in ft
3
/lb.
SOLUTION
Known:
Air executes a thermodynamic cycle consisting of three processes: Process 1–2, y 5 constant; Process
2–3, T 5 constant; Process 3–1, p 5 constant. Values are given for T
1
, p
1
, and p
2
.
TAKE NOTE...
To expedite the solutions of
many subsequent examples
and end-of-chapter problems
involving air, oxygen (O
2
),
nitrogen (N
2
), carbon dioxide
(CO
2
), carbon monoxide (CO),
hydrogen (H
2
), and other
common gases, we indicate
in the problem statements
that the ideal gas model
should be used. If not
indicated explicitly, the suit-
ability of the ideal gas model
should be checked using the
Z chart or other data.
c03EvaluatingProperties.indd Page 128 7/1/10 7:17:52 AM user-s146 c03EvaluatingProperties.indd Page 128 7/1/10 7:17:52 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
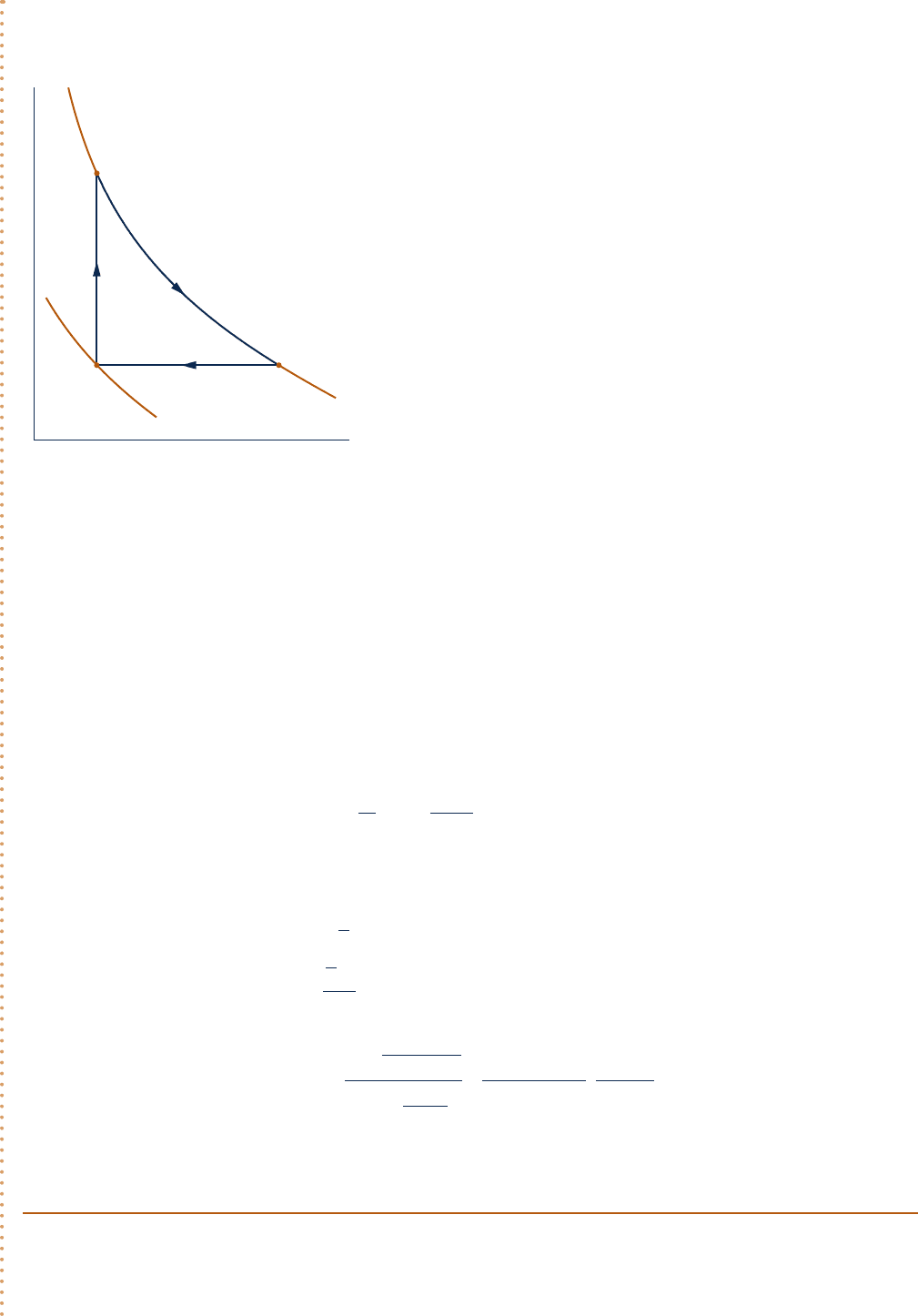
Find: Sketch the cycle on p–y coordinates and determine T
2
and y
3
.
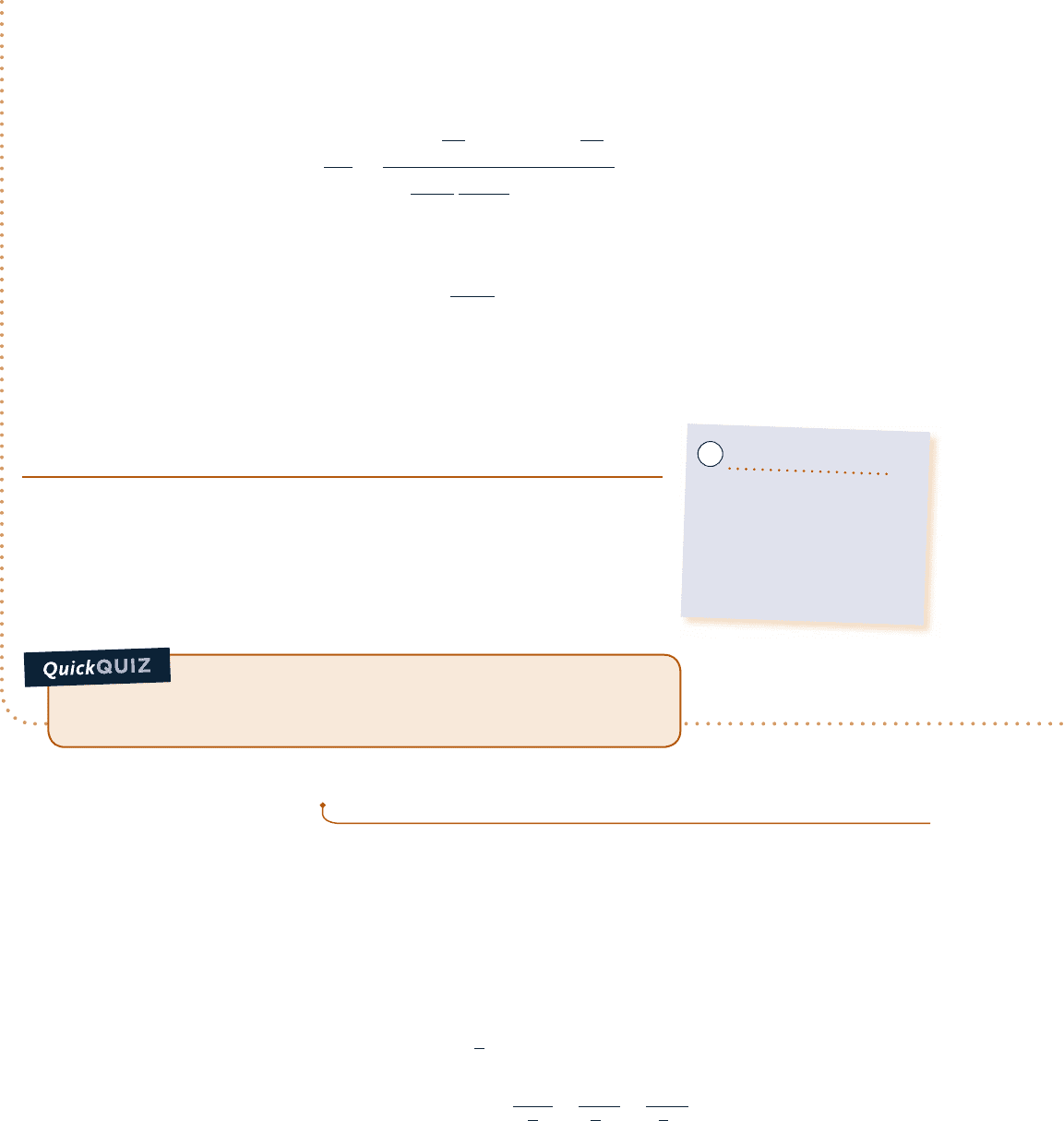
Schematic and Given Data:
Engineering Model:
1. The air is a closed system.
➊ 2. The air behaves as an ideal gas.
3. The piston is the only work mode.
2
1
3
p
1
= 1 atm
p
2
= 2 atm
T = C
v = C
p = C
1080°R
540°R
v
p
Fig. E3.8
Analysis:
(a) The cycle is shown on p–y coordinates in the accompanying figure. Note that since p 5 RT/y and tempera-
ture is constant, the variation of p with y for the process from 2 to 3 is nonlinear.
(b) Using py 5 RT, the temperature at state 2 is
T
2
5 p
2
y
2
y
R
To obtain the specific volume y
2
required by this relationship, note that y
2
5 y
1
, so
➋ y
2
5 RT
1
y
p
1
Combining these two results gives
T
2
5
p
2
p
1
T
1
5
a
2 atm
1 atm
b
15408R25 10808R
(c) Since py 5 RT, the specific volume at state 3 is
y
3
5 RT
3
y
p
3
Noting that T
3
5 T
2
, p
3
5 p
1
, and
R
5 R
/
M
y
3
5
RT
2
M
p
1
5
±
1545
ft ? lbf
lbmol ? 8R
28.97
lb
lbmol
≤
110808R2
114.7 lbf
/
in.
2
2
`
1 ft
2
144 in.
2
`
5 27.2 ft
3
/
lb
where the molecular weight of air is from Table A-1E.
3.12 Introducing the Ideal Gas Model 129
c03EvaluatingProperties.indd Page 129 5/27/10 7:57:05 AM user-f391 c03EvaluatingProperties.indd Page 129 5/27/10 7:57:05 AM user-f391 /Users/user-f391/Desktop/27MAY/Users/user-f391/Desktop/27MAY
130 Chapter 3 Evaluating Properties
3.12.3
Microscopic Interpretation
A picture of the dependence of the internal energy of gases on temperature at low
density (large specific volume) can be obtained with reference to the discussion of
the virial equations: Eqs. 3.30 and 3.31. As p S 0
1
y S q
2
, the force interactions
between molecules of a gas become weaker, and the virial expansions approach Z 5 1
in the limit. The study of gases from the microscopic point of view shows that the
dependence of the internal energy of a gas on pressure, or specific volume, at a
specified temperature arises primarily because of molecular interactions. Accordingly,
as the density of a gas decreases (specific volume increases) at fixed temperature,
there comes a point where the effects of intermolecular forces are minimal. The
internal energy is then determined principally by the temperature.
From the microscopic point of view, the ideal gas model adheres to several ideal-
izations: The gas consists of molecules that are in random motion and obey the laws
of mechanics; the total number of molecules is large, but the volume of the molecules
is a negligibly small fraction of the volume occupied by the gas; and no appreciable
forces act on the molecules except during collisions. Further discussion of the ideal
gas using the microscopic approach is provided in Sec. 3.13.2.
➊ Table A-1E gives p
c
5 37.2 atm, T
c
5 2398R for air. Therefore, p
R2
5 0.054,
T
R2
5 4.52. Referring to Fig. A-1, the value of the compressibility factor at
this state is Z
<
1. The same conclusion results when states 1 and 3 are
checked. Accordingly, py 5 RT adequately describes the p–y–T relation for
the air at these states.
➋ Carefully note that the equation of state py 5 RT requires the use of abso-
lute temperature T and absolute pressure p.
Is the cycle sketched in Fig. E3.8 a power cycle or a refrigera-
tion cycle? Explain. Ans. A power cycle. As represented by enclosed area
1-2-3-1, the net work is positive.
Ability to…
❑
evaluate p–y–T data using the
ideal gas equation of state.
❑
sketch processes on a p–y
diagram
✓Skills Developed
3.13 Internal Energy, Enthalpy, and Specific
Heats of Ideal Gases
3.13.1
Du, Dh, c
y
, and c
p
Relations
For a gas obeying the ideal gas model, specific internal energy depends only on tem-
perature. Hence, the specific heat c
y
, defined by Eq. 3.8, is also a function of temperature
alone. That is,
c
y
1T25
d
u
d
T
1ideal gas2
(3.38)
This is expressed as an ordinary derivative because u depends only on T.
By separating variables in Eq. 3.38
du 5 c
y
1
T
2
dT (3.39)
c03EvaluatingProperties.indd Page 130 5/19/10 8:32:32 PM user-s146 c03EvaluatingProperties.indd Page 130 5/19/10 8:32:32 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
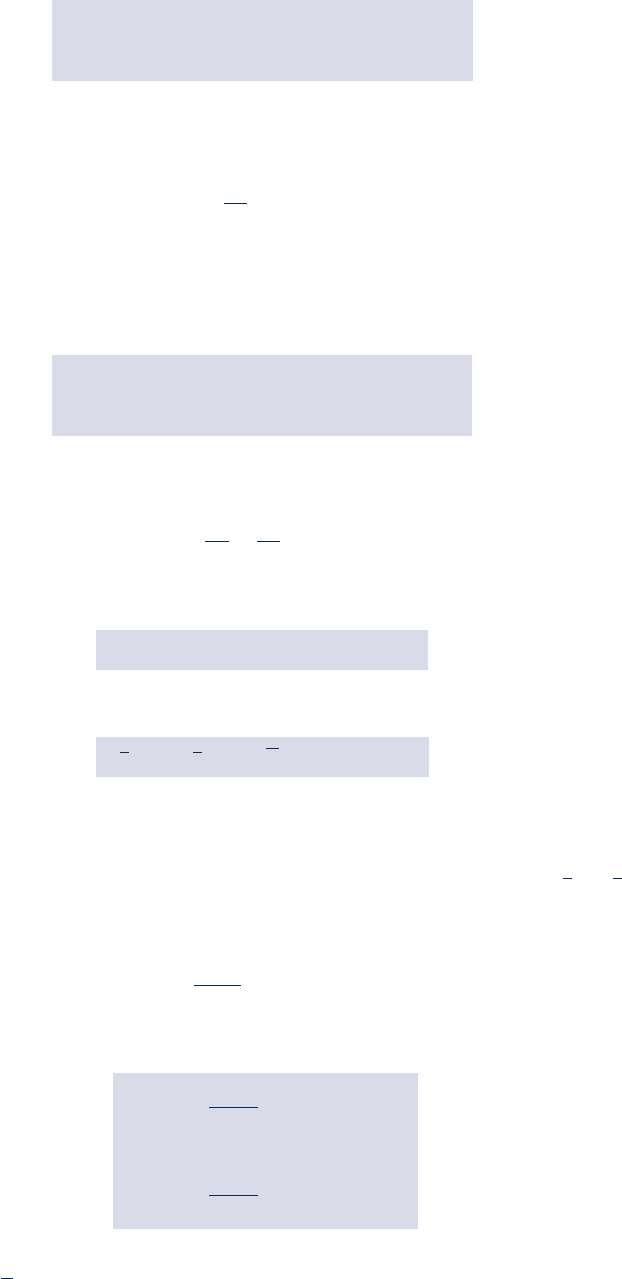
On integration, the change in specific internal energy is
u1T
2
22 u1T
1
25
#
T
2
T
1
c
y
1T2 dT
1ideal gas2
(3.40)
Similarly, for a gas obeying the ideal gas model, the specific enthalpy depends only
on temperature, so the specific heat c
p
, defined by Eq. 3.9, is also a function of tem-
perature alone. That is
c
p
1T25
dh
d
T
1ideal gas2
(3.41)
Separating variables in Eq. 3.41
dh 5 c
p
1
T
2
dT (3.42)
On integration, the change in specific enthalpy is
h1T
2
22 h1T
1
25
#
T
2
T
1
c
p
1T2 dT
1ideal gas2
(3.43)
An important relationship between the ideal gas specific heats can be developed
by differentiating Eq. 3.37 with respect to temperature
dh
d
T
5
du
d
T
1 R
and introducing Eqs. 3.38 and 3.41 to obtain
c
p
1
T
2
5 c
y
1
T
2
1 R
1
ideal gas
2
(3.44)
On a molar basis, this is written as
c
p
1T25 c
y
1T21 R
1ideal gas2 (3.45)
Although each of the two ideal gas specific heats is a function of temperature,
Eqs. 3.44 and 3.45 show that the specific heats differ by just a constant: the gas constant.
Knowledge of either specific heat for a particular gas allows the other to be calculated
by using only the gas constant. The above equations also show that c
p
. c
y
and c
p
. c
y
,
respectively.
For an ideal gas, the specific heat ratio, k, is also a function of temperature only
k 5
c
p
1
T
2
c
y
1
T
2
1ideal gas2
(3.46)
Since c
p
. c
y
, it follows that k . 1. Combining Eqs. 3.44 and 3.46 results in
c
p
1T25
kR
k
2 1
(3.47a)
(ideal gas)
c
y
1T25
R
k
2 1
(3.47b)
Similar expressions can be written for the specific heats on a molar basis, with R being
replaced by
R
.
3.13 Internal Energy, Enthalpy, and Specific Heat of Ideal Gases 131
c03EvaluatingProperties.indd Page 131 5/19/10 8:32:33 PM user-s146 c03EvaluatingProperties.indd Page 131 5/19/10 8:32:33 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
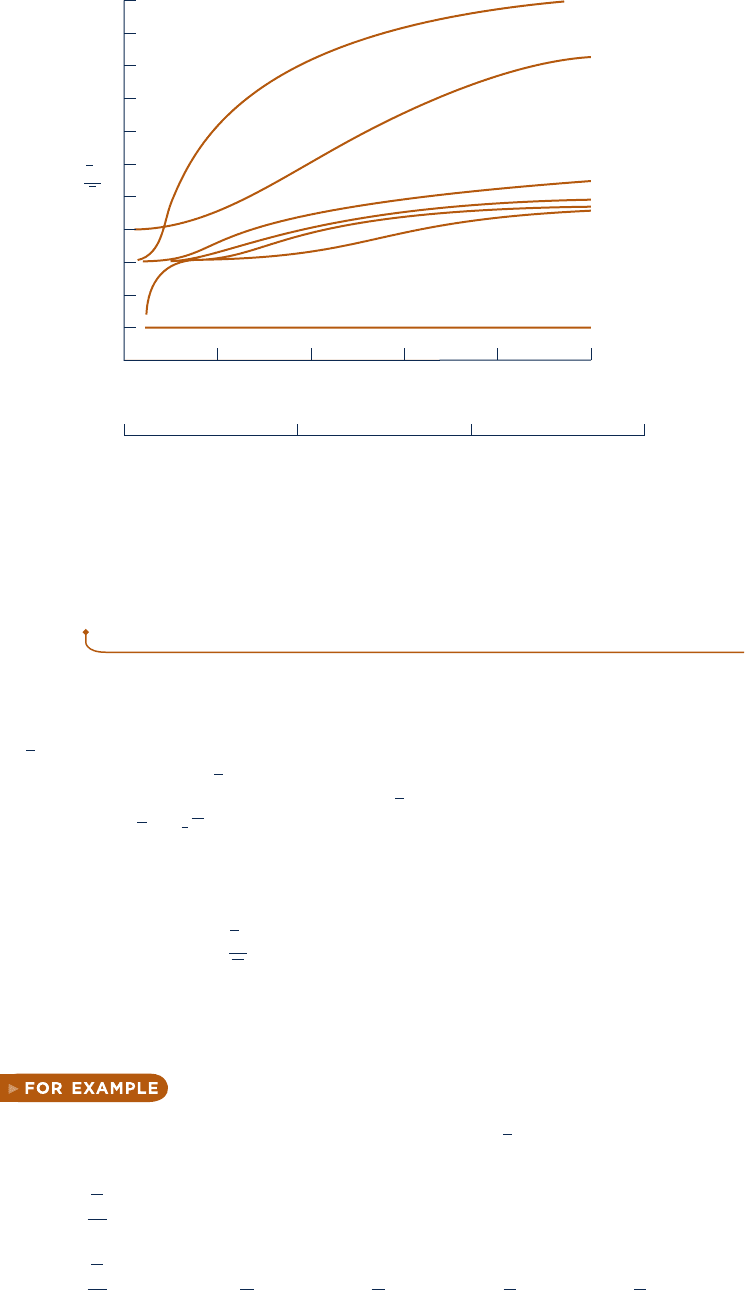
132 Chapter 3
Evaluating Properties
3.13.2
Using Specific Heat Functions
The foregoing expressions require the ideal gas specific heats as functions of tem-
perature. These functions are available for gases of practical interest in various
forms, including graphs, tables, and equations. Figure 3.13 illustrates the variation
of c
p
(molar basis) with temperature for a number of common gases. In the range
of temperature shown, c
p
increases with temperature for all gases, except for the
monatonic gases Ar, Ne, and He. For these, c
p
is constant at the value predicted by
kinetic theory: c
p
5
5
2
R. Tabular specific heat data for selected gases are presented
versus temperature in Tables A-20. Specific heats are also available in equation
form. Several alternative forms of such equations are found in the engineering
literature. An equation that is relatively easy to integrate is the polynomial form
c
p
R
5 a 1 bT 1 gT
2
1 dT
3
1 eT
4
(3.48)
Values of the constants a, b, g, d, and e are listed in Tables A-21 for several gases in
the temperature range 300 to 1000 K (540 to 18008R).
to illustrate the use of Eq. 3.48, let us evaluate the change in spe-
cific enthalpy, in kJ/kg, of air modeled as an ideal gas from a state where T
1
5 400 K
to a state where T
2
5 900 K. Inserting the expression for c
p
1T2 given by Eq. 3.48 into
Eq. 3.43 and integrating with respect to temperature
h
2
2 h
1
5
R
M
#
T
2
T
1
1a 1 bT 1 gT
2
1 dT
3
1 eT
4
2dT
5
R
M
c
a1T
2
2 T
1
21
b
2
1T
2
2
2 T
2
1
21
g
3
1T
3
2
2 T
3
1
21
d
4
1T
4
2
2 T
4
1
21
e
5
1T
5
2
2 T
5
1
2
d
7
6
5
4
3
2
0
0
1000
1000 2000 3000
2000 3000 4000 5000
c
p
R
Temperature, °R
Temperature, K
CO
2
H
2
O
O
2
CO
H
2
Air
Ar, Ne, He
Fig. 3.13 Variation of
---
c
p
/
----
R with temperature for a number of gases modeled
as ideal gases.
c03EvaluatingProperties.indd Page 132 5/19/10 8:32:34 PM user-s146 c03EvaluatingProperties.indd Page 132 5/19/10 8:32:34 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
