Lowenthal G., Airey P. Practical Applications of Radioactivity and Nuclear Radiations
Подождите немного. Документ загружается.

prompt gamma ray neutron activation analysis (Section 7.4.4) with the dual
transmission technology for ash and microwave methods for moisture.
7.2.2 Applications based on gamma ray backscatter
Backscatter gauges
In this section attention will be paid to the utilisation of g ray backscatter. A
number of applications are listed in Table 7.1.
Backscatter gauges are frequently used to monitor the levels of liquids in
tanks when transmission measurements are not practical (Figure 7.8(a)). The
intensity of backscattered radiation registered by the gauge depends primarily
on the bulk density of the material in the tank. It is clearly greater below the
liquid±gaseous interface than above.
The variation of the ef®ciency of single Compton backscatter (Figure
7.8(b)) with the angle of the scattered g ray and with the density of the
material in the tank is shown in Figure 7.8(c). The gauges are designed to
optimise the backscatter angle. The countrate response increases with in-
creasing density of the material and is enhanced by low-energy multiply
scattered radiation.
The correct detection of the boundary between liquids of different densities
depends also on the thickness of the tank wall. The walls attenuate both the
primary beam and the backscattered radiation so reducing the sensitivity of
the measurement. Backscatter measurements using g rays at normally avail-
able energies are limited to vessels with wall thicknesses less than about
30 mm steel equivalent or thinner still when using low g ray energies.
Backscatter techniques employing g rays are not particularly suitable for
locating liquid±liquid interfaces where the bulk densities of the two liquids
are similar. This is different for neutron backscatter gauges (Section 7.4.2)
which respond principally to differences in hydrogen concentrations. They
are usually much more sensitive than g ray detectors in de®ning the interface
between immiscible liquids (Figure 7.8(d)).
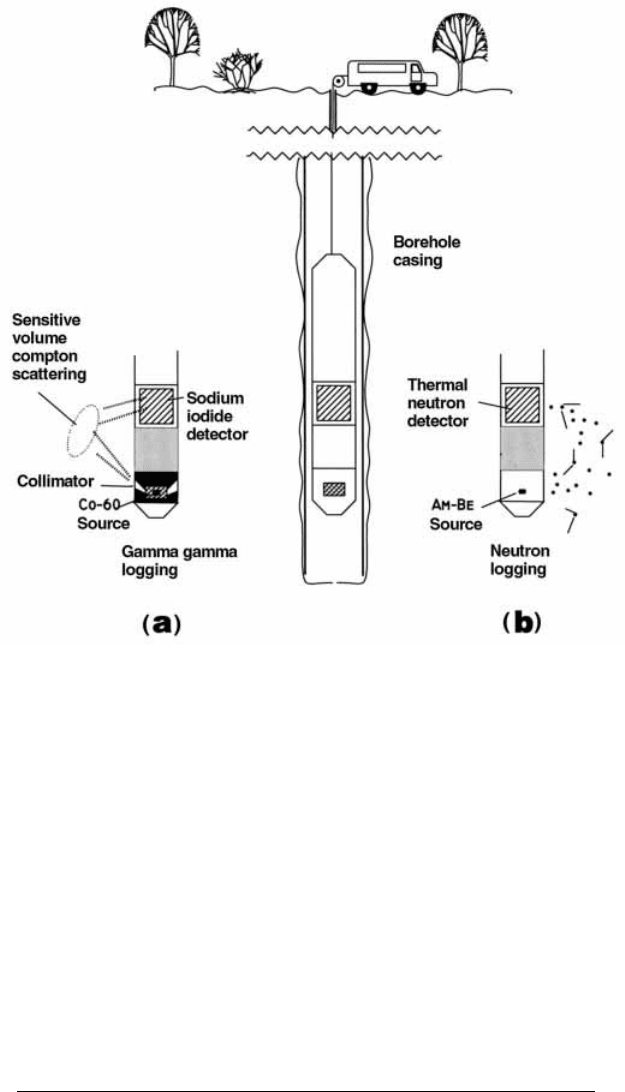
Borehole logging using backscattered g rays
A range of nuclear techniques, including g ray backscatter, complement
conventional methods in the comprehensive logging of boreholes (IAEA
1971, 1993). The essentials of a backscatter or g±g gauge, were introduced
above and are illustrated in Figure 7.9(a). As discussed above, the gauge
comprises a source and a detector separated by shielding to absorb directly
transmitted radiation.
7.2 Applications of gamma rays 201
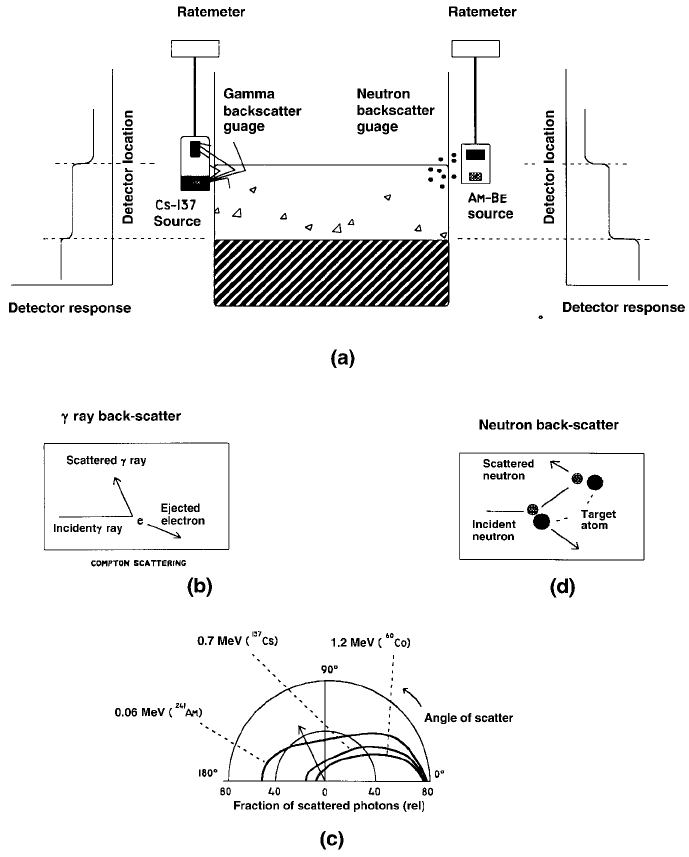
Industrial applications of radioisotopes and radiation202
Figure 7.8: Gamma and neutron backscatter gauges. (a) Application of g and n
backscatter gauges to measurements of liquid levels in tanks. (b) Compton back-
scatter. (c) Variation with angle of the ef®ciency of single Compton backscatter for
241
Am,
137
Cs and
60
Co gamma rays. (d) Neutron scatter by elastic collisions.
The response of g±g gauges depends primarily on the energy of the incident
radiation. At energies above the range 100 to 300 keV (depending on the
atomic number, Figure 3.7(a)), the Compton mechanism dominates and the
detector response depends on the bulk density of the surrounds. At lower
energies, the photoelectric effect becomes increasingly important and the
more so the higher the atomic number of the surrounding material. Hence,
the intensity of the low-energy component of the backscattered g radiation is
dependent on the effective atomic number Z
eff
, i.e. on the composition of the
surrounds.
In practice, measurements are made of the P
Z
ratio which is given by given
by:
P
z
= Intensity of Compton scattered radiation (E
g
> 300 keV)
Intensity of radiation in the low-energy region of the spectrum.
(7.3)
7.2 Applications of gamma rays 203
Figure 7.9. Borehole logging: (a) g±g and (b) neutron backscatter logging techni-
ques.
The numerator is a measure of the bulk density of the surrounding strata,
independent of the composition, whereas the P
Z
ratio is a measure of Z
eff
(i.e.
the composition), independent of density. Clearly detailed logging of the
geological strata requires a number of the complementary techniques
including neutron scatter which will be introduced in Section 7.4.2.
7.2.3 Applications based on X ray ¯uorescence
Introduction
Introductory information about the nature and properties of ¯uorescent
X rays was offered in Sections 3.9.1 and 3.9.2. X rays emitted from excited
atoms carry away the energy that is released when the atomic electrons move
from outer to inner atomic shells. The emitted energies can be predicted from
the differences in the average binding energies of the electrons in their shells.
Figure 3.16(a) shows a simpli®ed diagram of the electronic shells for the
element nickel (Z = 28).
X ray ¯uorescent spectra are characteristic of excited atoms and are
extensively used in analysing the composition of material for a wide range of
elements. Reference will be made in this section to X ray ¯uorescence (XRF)
analysis and to portable XRF gauges. X ray energies are listed in Table 3.2.
A more comprehensive listing has been posted on the Internet (see Chu et al.
(1999) and Table A3.1, Appendix 3).
X ray ¯uorescence analysis
X ray ¯uorescence analysis (XRF) is a technique for the analysis of a wide
range of elements without the requirement for chemical pre-treatment. The
sample is irradiated with an X ray beam of the required energy and the
characteristic ¯uorescent X rays are detected (Figures 7.10(a) and (b)).
An X ray generator with a monochromator is the usual source since
intensities are several orders of magnitude greater than the alternative radio-
isotope sources. Monochromatic X rays yield increased analytical sensitivity
by minimising the degree of overlap of the ¯uorescent X ray energies of
interest with unwanted peaks from scattered X rays. The ef®ciency of
emission of ¯uorescent radiation from an element reaches a maximum when
the excitation energy is close to, but just above the absorption edge of
interest.
There are basically two methods of detection: energy-dispersive X ray
spectroscopy (EDS) using high-resolution semiconductor detectors or wave-
length-dispersive X ray spectroscopy (WDS). Details are beyond the scope of
Industrial applications of radioisotopes and radiation204
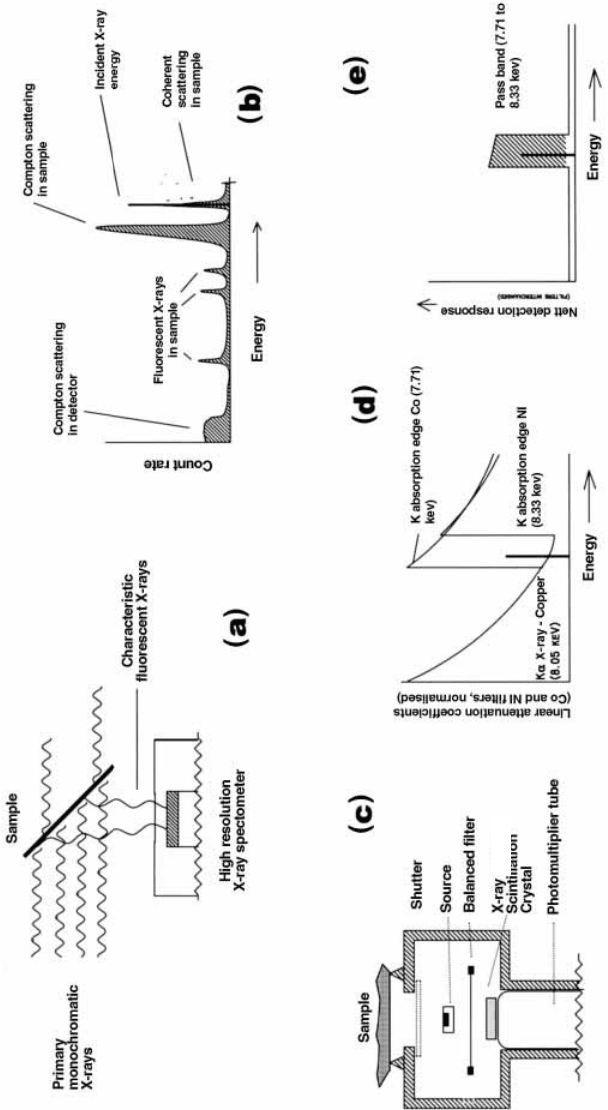
Figure 7.10. X ray ¯uorescence analysis. (a) Schematic representation
of XRF analysis system. (b) Features of an XRF spectrum
(after Jaklevic
et al., 1977). (c) Schematic diagram of the head of a portable XRF analyser
showing the location of the source, the
sample, the balanced ®lter and the scintillation crystal. (d) The linear attenuatio
n coef®cients of the balanced cobalt and nickel ®lters
in the vicinity of the copper K
a
X ray emission. (e) The pass band, i.e. the change in the detector response
when the balanced cobalt
and the nickel ®lters are interchanged.
Nal(Tl)
this text. Readers are referred to Lachance and Chaisse (1995) for a
comprehensive account of XRF.
Portable X ray ¯uorescence gauges
Portable XRF analysers have been developed for ®eld applications by
replacing the X ray generator and monochromator with radioisotope sources
and X ray ®lter systems. The lower intensity is offset by portability. The
principle is here illustrated by the assay of copper. Two factors will be
considered: (a) the choice of an isotopic source to optimise the emission of the
copper KX rays and (b) the use of balanced ®lters to minimise interference by
unwanted peaks.
(1) Excitation energies: The X ray energy emitted by the isotopic source and
intended to trigger the copper KX rays should be greater than, but as close as
practical to 9.0 keV, the energy of the K absorption edge of copper. A number
of radionuclides emitting X rays or g rays at a suitable energy are commercially
available and are listed in Table 7.4. In this case one can use either
238
Pu
(T
1/2
= 87 y), emitting X rays in the range 12 to 17 keV or
109
Cd (T
1/2
= 1.2 y)
emitting X rays in the range 22 to 25 keV.
(2) Balanced ®lters: Although the correct choice of the excitation source will
maximise the yield of the K ¯uorescent X rays, interference from other elements
will also be present. Interfering X ray energies are not easily identi®ed because
portable XRF analysers use NaI(Tl) detectors which are of relatively low energy
resolution. They cannot resolve individual KX rays but only show their
average. Also, the entrance window of the detector must be thin enough to
transmit the required X ray energies with suf®cient intensit y.
A portable X ray ¯uorescence (XRF) analyser is shown schematically in
Figure 7.10(c). In this design, pairs of balanced ®lters are used to minimise
the effects of extraneous emissions. When copper is monitored, cobalt and
nickel ®lters are used.
The measurement principle is illustrated in Figure 7.10(d) which shows the
attenuation for cobalt and nickel ®lters as a function of energy near the
copper KX ray emission. The attenuation factors have been normalised by
allowing for differences in the thicknesses of the ®lters. The K absorption
edge for cobalt (7.71 keV) is less than, and the K absorption edge for nickel
(8.33 keV) is greater than that of the average energy of the ¯uorescent copper
KX ray (8.05 keV).
The measurement involves recording the difference in countrates when one
®lter is replaced by the other. This de®nes a narrow pass band (7.71 to 8.33
keV) which includes the copper KX ray energy (Figure 7.10(e)). The relative
thicknesses of the two ®lters are adjusted so that the attenuation on either
206 Industrial applications of radioisotopes and radiation
side of the pass band is balanced. By using the balanced ®lters, a good
measurement of the level of copper is obtained in spite of potential inter-
ference from other elements in the sample which would not be resolved by the
sodium iodide detector. A list of balanced ®lters of elements frequently used
for KX ray detection is reported by Charlton (1986, Table 14.2).
Applications to the mineral processing industry
Reference was made above to the role of nucleonic density gauges in the real
time analyses of the levels of valuable minerals in process streams. The
identi®cation of these levels is often made using radioisotope X ray ¯uores-
cence techniques. Since the complex minerals processing stream is the target,
a complex spectrum of ¯uorescent X rays is produced.
In many industrial environments, the challenge is to monitor the desired
economically important elements using ruggedised systems based on sodium
iodide detectors which are of limited resolution. An example of such an
approach is the use of balanced ®lters described above. A range of more
elaborate techniques has been developed for applications in the minerals
industry (see Watt, 1972, 1973).
7.3 Scienti®c and industrial applications of beta particles and electrons
7.3.1 Attenuation of beams of beta particles and electrons
Whereas g rays are uncharged electromagnetic radiations, b radiations are
charged particles interacting with matter primarily through Coulomb interac-
tion with the outer electrons of its atoms (Section 3.3.1). The range, t cm, of a
beam of mono-energetic electrons depends principally on the energy of the
electrons and the density, r g/cm
3
, of the material in which they move. It was
shown in Section 3.3.5 that the product t6r g/cm
2
known as surface density,
has the useful property of being only weakly dependent on the Z number of
the absorbing element. With b particles having short ranges in solids (Figure
3.2(b)), their attenuation is used for the measurement of the thickness of ®lms
in the paper, plastics and rubber industries. Comprehensive listings of the
ranges of electrons have been published in regular reports (Berger and
Seltzer, 1964) and on the Internet (Berger, 1999).
Frequently used commercially available b particle sources include
85
Kr
(T
1/2
= 10.73 y, E
b
(max) 672 keV) and
147
Pm (T
1/2
2.62 y, E
b
(max) 225 keV)
(Table 8.2). The detectors are saturation ionisation chambers ®lled with
argon at two or more atmospheres pressure to increase their ef®ciency
(Section 6.3.4). Ionisation chambers are used because they can cope with
7.3 Applications of beta particles and electrons 207
high particle ¯uxes without the need for dead time corrections (Section
4.5.2).
Applications in paper manufacture
Nucleonic gauges using such sources are ®tted routinely to new paper
manufacturing plants and are often retro®tted to older units. They may be
used to continuously monitor the thickness of paper at speeds up to 400 m/s.
Optimum ranges depend on the source chosen (0.03 to 1 g/cm
2
with
85
Kr, and
0.015 to 0.175 g/cm
2
with
147
Pm).
On-line processing of the output data from the detectors allows the
collection and display of short-term trends and overall average variations in
the thickness of the paper. The output data may be used to control the speed
and inclination of the rollers to ensure that the paper thickness always
remains within tolerance. Using microwave or infrared techniques, the
moisture level is monitored to about 0.2% and controlled by automatically
varying the amount of evaporative heating. Through the use of these control
systems, the paper is manufactured within tighter tolerances than would
otherwise be possible so gaining a higher quality product and a reduction in
the use of materials and energy.
7.3.2 Industrial applications of beta particle backscatter
Assuming saturation thickness (Section 3.3.3), the ef®ciency of the back-
scatter of b particles increases with the energy of the incident radiation and
with the electron density i.e. the effective Z number of the backscattering
material (Figure 3.5).
While b particle transmission techniques are extensively used in industry
to measure the thickness of paper and other ®lms, b particle backscatter
methods are well suited to measurements of the thickness of thin coatings
on substrates that are thick enough for saturation backscatter (Section
3.3.3). The radiation source in a backscatter gauge is a pure b emitting
isotope with an energy chosen to suit the application. Examples include
63
Ni,
147
Pm and
204
Tl (Table 8.2). A practical gauge incorporates a micro-
processor to convert the reading into a meaningful measure of coating
thickness.
Since the ef®ciency of backscatter from a material is proportional to its
electron density, the best de®ned applications are those in which a material of
high atomic number is laid on one of preferably much lower atomic number
or vice versa (Charlton, 1986, Ch. 14). Applications include the monitoring
of the thickness of electroplated gold and the thickness of plastic coatings on
Industrial applications of radioisotopes and radiation208
metals (see Table 7.5); suitable sources for these examples are
204
Tl and
63
Ni
respectively.
7.3.3 Special applications: electron microscopy
Knowledge of the relationships between the properties, structure and com-
patibility of materials under a wide range of conditions underpins many of
the processing, manufacturing and heavy engineering sectors of industry.
Electron microscopy, which involves the application of many of the scienti®c
principles discussed in this book, has been used to advance knowledge in
these areas. In simple terms, an electron microscope comprises a source of
electrons and a series of electro-magnets, which perform functions similar to
that of lenses in optical microscopes.
The fundamental difference between optical and electron microscopes is
the mechanism of formation of the contrasts resulting in the image. In light
optics, images result from differences in absorption of the light illuminating
the object; in electron microscopy they arise from the complex processes of
electron scattering and diffraction.
In both types of instruments, the Rayleigh criterion requires that the limit
of resolution, d, is a function of the incident wavelength given by d =kl/a
where k is a constant, l is the wavelength of the incident radiations and a is
the aperture of the microscope, all in consistent units. For optical micro-
scopes, the resolution lies in the range 0.2 and 0.5 mm (2000 to 5000 A
Ê
),
leading to a maximum magni®cation of around 10006.
The wavelengths of electrons decrease as their energies increase i.e. with
increasing accelerating voltage. Electron wavelengths are very short, only
0.09 A
Ê
at 20 keV and 0.025 A
Ê
at 200 keV, and so substantially less than the
average interatomic spacings which are about 2 A
Ê
. With modern high-
resolution techniques, well maintained and properly aligned transmission
electron microscopes can resolve at the atomic scale. However, due to a
number of instrumental effects, the resolution for scanning electron micro-
scopes is limited to between 50 and 200 A
Ê
, which corresponds to magni®ca-
tions of about 100 0006.
The interaction of higher energy electrons (> 5 keV) with the atoms of the
specimen leads to the generation of X rays. These comprise both bremsstrah-
lung (Section 3.8.1) and ¯uorescent X rays (Section 3.9.1). The latter are used
to map the distribution of elements in the sample. In addition, structural
information may be obtained through the analysis of electron diffraction
patterns. Readers seeking further information are referred to works by
Hunter et al. (1993), Williams and Carter (1996) and Watt (1997).
7.3 Applications of beta particles and electrons 209
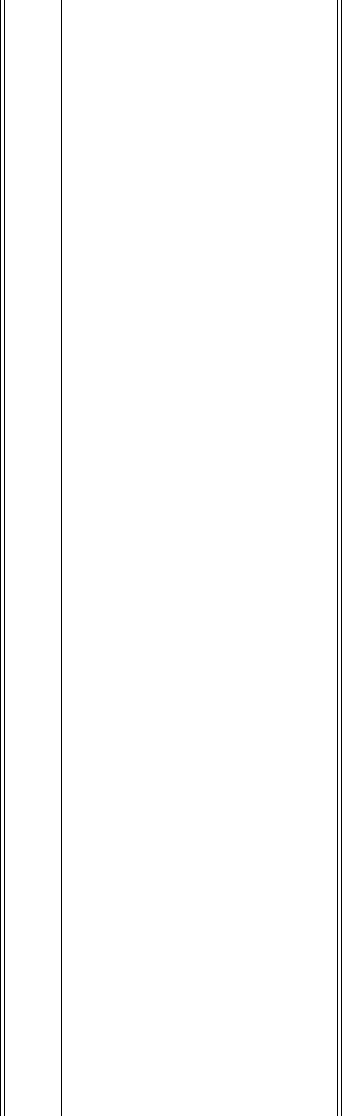
Table 7.5.
Industrial applications of beta particles and electrons.
Property of the Application
Example
Reference
Radiation
Transmission
Thickness measurements Thickness control in paper manufacturing industry
based on the attenuation of transmitted
85
Kr b
Section 7.3.1
particles
Backscatter
Thickness measurements
Section 7.3.2
of coatings
Absorption
Chemical processing; Irradiation by electron beams or the derived
Sterilisation;
bremsstrahlung is an alternative to gamma ray
Radiation curing. treatment. Electron beam irradiation
complements Section 7.6
UV as a curing agent in the printing and coating
industries
Special applications Electron microscopy Investigation of
materials characteristics, composition
and structure
Section 7.3.3
Note: The properties of beta particles are discussed in Section 3.3 and the
decay data for sealed beta particle sources are presented in
Table 8.2.
