Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.

8 Chapter 1
1.2.4. Strength
Plant operation will be adversely affected as pressure drop increases through the
catalyst bed. This may be due to:
• Dust formed from catalyst disintegration during changing and operation.
• Entrained liquid cementing the catalyst.
• Entrained solids blocking the bed.
• Collapsed beds following mechanical damage to bed supports.
• Carbon formation from organic feedstocks.
These problems generally affect the temperature profiles in the bed and,
possibly, the overall reaction. The catalyst must be strong enough to resist vari-
ous forms of regeneration or reactivation.
1.3. CATALYST PRODUCTION
Catalysts are produced in different ways depending on the chemical formulation
and the severity of the chemical process in which they will be operated. The
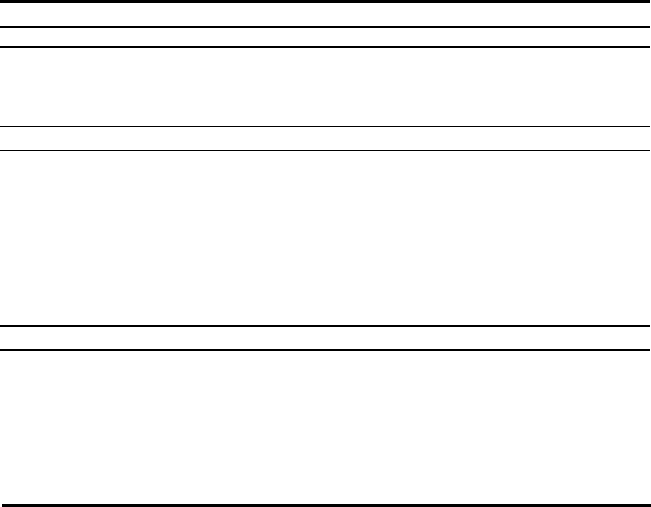
usual methods are listed in Table 1.5. Figures 1.2 and 1.3 show typical catalyst
production facilities (See also Table 1.9 where some unit operations used in cat-
alyst manufacturing are listed).
TABLE 1.5. Preparation and Application of Industrial Catalysts.
Preparation Application Catalyst
Impregnation Suitable supports impregnated with soluble
salts of the catalytic metals which are de-
composed to oxides and reduced before use.
Catalysts containing small
amounts of precious metals or
easily impregnated amounts of
base metals.
Precipitation Carbonates/hydroxides of catalytic metals
precipitated, decomposed and pelletted, ex-
truded or granulated before reduction.
Catalysts containing high con-
centrations of base metals,
which are required in a particu-
lar physical form.
Fusion Metal oxides fused and chill cooled before
crushing and sieving to required size.
Only used in relatively few
catalysts.
Metals Metal catalysts alloyed with a metal soluble
in alkali. Catalyst active after removing sol-
uble material. Alternatively, thin wires of
catalytic metals can be woven into fine mesh
and used directly. Metals occasionally used
directly in granule form.
Only used in special applica-
tions.

Figure
up fro
m
Ed. by
M
sion of
M
Fig
u
1.2. Semi-tech
n
m
laboratory to f
u
M
. V. Twigg,
W
M
. Twigg.
u
re 1.3. Typica
l
n
ical unit for c
a
u
ll-scale manuf
a
W
olfe Publishin
g
l
production lin
e
a
talyst preparati
o
a
cture. Reprinte
d
g
, LTD., Londo
n
e
for manufactu
r
Industrial
o
n, an intermed
i
d
from Catalyst
H
n
, England, 198
r
e of catalysts b
y
Catalysts
i
ate stage in sc
a
H
andboo
k
, 2
nd
e
9,
b
y kind per
m
y
precipitation.
9
a
le-
e
d.,
m
is-
10 Chapter 1
Many of the earliest catalysts were based on natural products or porous re-
fractory materials that were available commercially, but improved catalysts were
especially made as large-scale processes developed. Table
1.6 shows some of
the materials that have been used as support.
The principal objective in catalyst production, however, has usually been to
make a suitable catalyst for any large-scale process by the most reasonable eco-
nomical procedure. Catalysts with a specific chemical composition have been
established and appropriate standards for such physical properties as particle
size and strength have been developed for most commercial processes. Target
specifications provide for reasonably predictable operation at an acceptable
pressure drop when used in standard plant equipment. After the initial period of
deactivation, provided that the decline of catalyst performance is very slow and
predictable, a process can be designed that is economic over the life span of the
catalyst. A typical catalyst specification is shown in Table 1.7.
The most frequently used methods to produce catalysts are precipitation and
impregnation. In both processes the catalyst precursors are usually converted to
oxides by heating and the powders converted to solid granules or pellets. Cata-
lysts often contain promoters that are added during the preparation stages. If
TABLE 1.6. Catalyst Supports.
Material Function
Essential properties of support:
Inert Should not react with the active catalyst or take part in reaction.
Strong Should not disintegrate during handling or use; low attrition loss.
Porous Disperses the active catalyst to increase the activity and reduce
the cost of expensive material.
Early supports
Asbestos Contact process.
Pumice Deacon process, hydrogenation catalyst supports.
Kieselguhr (infusorial earth) Fat hardening, hydrogenation catalyst support.
Bauxite/titanium dioxide Dehydration reactions, catalyst support and cracking catalyst.
Carbon Support for precious metals.
Metal salts, e.g., MgSO
4;
MgCl
2
Contact process; olefin polymerization.
Quartz lumps Used as an inert support for catalysts and also as a physical
support at the bottom of a catalyst bed.
Modern Supports
α-Alumina Gamma and
α
-alumina used as a catalyst support in many
reactions.
Silica Produced as a pure catalyst support in a number of forms.
Activated carbon Still used as a catalyst support in some processes.
Silica/alumina Only used in special applications now that it has been replaced
by zeolite in cracking reactions.
Cordierite Used as preformed monolith in automobile exhaust treatment
and other similar applications.
Industrial Catalysts 11
TABLE 1.7. Typical Catalyst Specifications.
Property Test
Chemical analysis
Major components: wt% (either maximum/minimum level or acceptable
range.)
Impurities: wt% or ppm (maximum permitted level.)
Loss on ignition: wt% (sample calcined to a loss-free basis at an appropriate
temperature.)
Physical properties Particle shape: pellets, granules, rings, extrusions.
Particle size: length, diameter.
Bulk density: kg.liter
-1
; lb.ft
-3
Crushing strength: lb or kg nominal.
Micromeritics Surface area: m
-2
g
-1
Mercury density: g.cm
-3
Helium density: g.cm
-3
Mean pore radius: Å
Testing is usually done on a bulked representative sample from the daily production.
necessary, a support may be included with the solutions used during
precipitation, as well as being impregnated directly with oxide precursors.
Many catalysts can be made by either of the two methods. Precipitation is
usually chosen if a support is not porous enough for sufficient active metal to be
loaded by impregnation. On the other hand, low concentrations of expensive
precious metals are impregnated on to suitable supports particularly when they
can be deposited on the surface of the support for greater efficiency. Conditions
must be carefully controlled during catalyst production to give consistent
quality. The checks carried out during all stages of production are listed in Table 1.8.
TABLE 1.8. Routine Testing During the Production of an Industrial Catalyst.
Test Properties
Routine chemical analysis
(products and raw materials)
Major metal components, metal impurities, well-mixed phases.
Crushing strength Compression strength between faces (vertical) or across diameter
(horizontal).
Attrition/tumbling loss Dust formed during rotation in a tube under standard conditions.
Micromeritics Surface area, helium and mercury density, pore volume, mean
pore radius.
Particle size Average length of pellets, average diameter of spheres, average
length of extrusions.
Particle size distribution Proportion of required, undersize, and oversize particles.
Particle porosity Internal volume available during impregnation and operation.
Bulk density Indication of pelletting or granulation efficiency.
Catalyst activity and stability Determines preliminary reduction and operating efficiency.
12 Chapter 1
Porosity and surface area are probably the most important properties of a
catalyst as they control access to the active sites of the catalyst. Pore size and
surface areas can be moderated in a number of ways to control selectivity and
access of large molecules to the catalyst. It is interesting to remember that 500 g
of catalyst with a surface area of 100 m
2
.g
−1
has a total area of about 12 acres. A
catalyst charge of 40 tonnes therefore has a total surface of almost 1 million
acres. An amazing figure!
1.3.1. Precipitation
Aqueous solutions of the metal salts, usually nitrates or sulfates, are precipitated
with an alkali such as sodium carbonate. Ammonium carbonate can be used to
avoid residual sodium impurity, but it is relatively expensive. Occasionally
precipitation conditions are controlled to form complex catalyst precursors and
higher-activity products. If necessary, any support material can be added as a
powder before the alkali is added.
When the precipitate has formed and settled, it is filtered and carefully
washed before drying. Dried mud is then calcined at a temperature in the range
300
0
– 450
0
C to decompose hydrates and carbonates. The calcined mud can then
be grounded to powder and densified with a suitable lubricant before being
pelletted. Many important practical details are involved in precipitating
catalysts. The following are some points to remember:
• It is necessary to precipitate rapidly and maintain a uniform pH. This pro-
duces small active crystallites and well-mixed oxides in the finished
catalyst. Under these conditions specific compounds form such as those
described by Feitknecht, with the composition (M
2+
)
6
(M
3+
)2 (OH)16 (CO3)
4H2O. The Adkins catalyst, copper/ammonium chromate, is another
example of applying a specific precipitation procedure.
• Slow precipitation of metals from solution is possible by the slow
hydrolysis of urea or nitrite at about 90
0
C. This procedure is quite slow
and using it may be expensive for many catalysts.
• When preparing a nickel oxide/kieselguhr catalyst, reaction between the com-
ponents leads to the formation of nickel silicates, which are difficult to
reduce. This can affect operation and the catalyst may require prereduction
before use. The addition of a promoter such as copper oxide allows reduction
at a lower temperature.
• When calcining chromium catalysts in air any Cr
3+
is oxidized to Cr
6+
at
temperatures exceeding 200
0
C. Further calcination up to about 450
0
C
reduces the Cr
6+
content to a practical level and avoids an exothermic
reduction reaction in the plant.
Industrial Catalysts 13
1.3.2. Impregnation
Preformed, absorbent supports are uniformly saturated with a solution of the
catalytic metals. Several impregnations may be needed to obtain the required
metal loading. Supports must be strong enough to be immersed directly into the
solution and any dust forming should not contaminate the catalyst surface. To
avoid these difficulties, the supports can be sprayed with just enough solution to
completely fill the pores. At this point the support suddenly appears to be wet
and the procedure is known as incipient wetness. In some cases, when the
support has been impregnated, the metals may be precipitated by immersion in a
second solution.
After impregnation catalysts are carefully dried and most are calcined
before use to decompose the metal salts to oxides. Care is necessary to avoid
high concentrations of metals forming at the support surface as the solution
evaporates. Most types of supports can be used to produce impregnated
catalysts, although alumina or silica, in various forms, is usually chosen. The
support should not, of course, react with the metal solution. For example, if the
support is soluble in acid, there is a possibility of re-precipitation in an
undesirable form.
1.3.3. Other Production Methods
Many other procedures have been used to produce catalysts:
• Platinum/rhodium alloy gauze is used as a catalyst in the selective
oxidation of ammonia during nitric acid production and in the production
of hydrogen cyanide. The wire in the gauze is only a few thousandths of
an inch in diameter woven at 80 wires per inch. Several layers of gauze,
up to about 8 ft in diameter, are used.
• Silver granules are used to oxidize methanol to formaldehyde. Raney
nickel is produced by leaching aluminum from a nickel/aluminum alloy
with alkali solution. Not all of the alumina is removed and the catalyst
may be regenerated a number of times by alkali treatment.
• Mixtures of natural magnetite and various promoters are melted in a
furnace at about 1600
0
C and chill cooled by casting on a flat surface. The
catalyst can be crushed and separated into appropriate size ranges before
use in ammonia synthesis or Fischer–Tropsch processes.
• In large modern ammonia plants the synthesis catalyst is often used in a
pre-reduced form. The catalyst is carefully reduced in a mixture of
hydrogen and nitrogen and then re-oxidized with a mixture of oxygen and
nitrogen. Less than 20% of the iron is re-oxidized, making plant start-up
14 Chapter 1
much easier and ensuring that the catalyst is not pyrophoric before use.
Some nickel oxide catalysts supported on silica are also pre-reduced.
1.4. CATALYST TESTING
Industrial catalysts must conform to a strict specification and physical and chem-
ical properties are measured at all stages of production. The tests most often
included in catalyst specifications are listed in Table 1.7.
1.4.
1. Physical Tests
It is most important that the catalyst be strong enough to resist breakage and
attrition. Fixed bed and tubular reactors are carefully filled with catalyst to
ensure that the pellets or granules are not damaged and pack with a uniform
density.
Strength is particularly important in processes in which catalysts are
circulated continuously between the reactor and a regenerator. In the fluid
catalytic cracking process significant daily additions of catalyst must be made to
compensate for losses through attrition as well as catalyst deactivation.
1.4.2. Chemical Composition
Careful checks on the chemical composition of both raw materials and products
are required to ensure that the specification is achieved. It is important to
confirm that the desired chemical compounds are formed with the required
crystalline form and particle size. It is very easy for the pH of the solution to
change during precipitation reactions and influence the composition of the
precipitate.
Elemental analysis of the bulk components in a catalyst is measured by X-
ray fluorescence (XRF), which has now replaced traditional wet analysis. If
required, electron probe analysis can also provide information on the
distribution of elements in a catalyst particle. Bulk phases in a catalyst and
crystallite size are determined by X-ray diffraction (XRD), as either a routine
check or a diagnostic procedure to examine catalysts damaged during operation.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA)
with either air or inert atmospheres show weight changes or heat evolution as
catalyst samples are heated and intermediate chemical compounds decompose.
The tests are therefore useful in providing information on temperature-related
phase changes at different stages of catalyst preparation. Tests also show how
discharged catalysts react during regeneration or oxidation. Temperature
Industrial Catalysts 15
programmed reduction (TPR) in hydrogen is used to investigate similar changes
during catalyst reduction over the appropriate temperature range. These
procedures are widely used in catalyst development and routine testing.
Many other tests are used to measure the physical and chemical properties
of industrial catalysts during development and routine examination. These are
fully described in other publications but are summarized here in Tables 1.9 and
1.10.
1.
4.3. Activity Testing
Activity measurements have been important ever since industrial catalysts were
first introduced. BASF carried out some 20,000 tests during the production of a
successful ammonia synthesis catalyst. Until the 1960s, however, when many
new catalytic processes were developed, laboratory activity tests were fairly
crude. It was only possible to screen different samples and obtain relative
activities before new catalysts reached the semitechnical plant stage and
commercial trials were considered possible. More recently, new testing
equipment has been able to simulate plant conditions and provide accurate
kinetic information for reliable design calculations.
TABLE 1.9. Physical Testing of Industrial Catalysts.
Test Procedure
Chemical analysis X-ray fluorescence (XRF) Samples emit secondary X-rays
following bombardment with hard X-rays which allow complete
elemental analysis.
Particle size Simple measurement of solid particles or size grading of powders.
Crushing strength Determined by compressing catalyst particles between anvils or by
bulk crushing the catalyst in a standard container.
Bulk density Packing weight per unit volume of loose or dense packed catalyst
particles.
Attrition loss Dust formed after the rotation of catalyst for a fixed period in a
standard cylinder.
Crystalline phases X-ray diffraction (XRD) identifies crystalline compounds by
reference to standard tables. The proportion of each phase present
in the sample can be calculated.
Crystallite size Estimated from X-ray diffraction line broadening as crystallite size
decreases.
Surface area/pore size Calculated from the volume of a gas monolayer adsorbed by the
catalyst and the known area covered by a gas molecule. Pore
volume can be calculated from the helium density (helium fills the
pores) and the mercury density (mercury does not fill the pores).
The average pore radius assuming cylindrical pores is calculated
as twice the pore volume divided by the surface area.
16 Chapter 1
Thermogravimetric analysis
(TGA)
Measures the weight loss as catalyst composition changes at
increasing temperature with oxidizing or inert atmospheres.
Differential thermal analysis
(DTA)
Measures the exo-or endothermal temperature changes taking place
as catalysts are heated in oxidizing or inert atmospheres.
Temperature programmed
reduction
Measures the reducibility of oxides in catalyst samples in reducing
atmospheres.
There are several steps during the catalytic reaction before a gas molecule is
available for reaction at the active surface. First, it must (a) pass through the gas
film surrounding the catalyst particle, (b) diffuse through the catalyst pores and
reach an active site, and (c) adsorb on the active site to react with adjacent
molecules. Products then (d) desorb from the active site, (e) return through the
catalyst pores to the catalyst surface, and (f) re-enter the gas phase.
This process is dynamic and may depend on the reacting molecules moving
from site to site until reaction takes place and products can desorb. Under ideal
conditions, there would be no film or pore diffusion limitations but, practically,
these are often encountered in catalytic reactions, particularly when large rings
or pellets operate at a relatively low linear velocity.
Simple screening tests can be developed for most reactions to compare the
activity of different catalysts. It is important, however, to standardize operating
conditions and to operate well away from equilibrium conversion, to obtain the
most useful results. To avoid all diffusion limitations, catalyst samples are
normally tested at a high linear velocity with small crushed particles. Test units
operate with pure gas mixtures and the effects of typical poisons must be
considered in separate tests. Until the 1960s, the screening tests operated at
atmospheric pressure and compared the performance of new catalysts with an
accepted standard at constant space velocity.
TABLE 1.10. Chemical and Structural Analyses of Industrial Catalysts.
Test Result
Electron microscopy Used to study the surface structure and composition of
catalysts and has extended the use of optical microscopy in
determining the characteristics of particles.
Scanning electron microscopy
(SEM)
Electrons scan the surface of the sample and give a magnifi-
cation of 20,000–50,000. It can focus on sizes down to 5
nm. This gives crystallite shape, size, and size distribution.
Transmission electron microscopy
(TEM)
Higher magnification and resolution possible to give three-
dimensional images of crystallites down to 0.5 nm and
changes during operation.
Electron spectroscopy for chemical
analysis (ESCA) better known as
X-ray photoelectron spectroscopy
(XPS)
Can analyze for all elements and atomic electron binding
energies to give structural data and compound types in
surface layers.

Industrial Catalysts 17
Auger spectroscopy (AES) Elemental analysis of surface layers.
Secondary ion mass spectroscopy
(SIMS)
Elemental analysis of surface layers.
High-resolution electron energy-
loss spectroscopy (HREELS)
Types of chemical bond present.
Atmospheric pressure test units cannot give the information required for
modern catalyst and process development and have been replaced with high
pressure micro-reactors. To avoid diffusion limitations, micro-reactors operate at
very low conversions, under isothermal conditions, using small quantities of
crushed catalyst. Experimental catalysts are usually tested in the same process
conditions over a range of gas rates to provide useful design information. The
ratio of space velocities measured at the same conversion gives the relative
activity of different catalysts and an indication of the catalyst volume needed to
give the same performance. It is also possible, when using pulsed gas flow at a
constant space velocity and process conditions, to measure the catalyst
selectivity in a reaction over an appropriate temperature range.
Knowledge of reaction kinetics is required to enable engineers to design a
catalytic reactor. To obtain this information it is usual to use full-size catalyst
particles over the total range of plant operating conditions to determine an
accurate rate of reaction. This became possible by the introduction of continuous
stirred tank reactors. These give results directly for conditions that are almost
uniform throughout the reactor with none of the gradients found in micro-
reactors. Two popular types of high-pressure reactors are the Carberry reactor,
in which the catalyst is held in a cross-shaped basket and rotated, and the Berti
reactor, which recycles gas through a fixed bed of catalyst. Several catalytic
reactors are listed in Table 1.11.
TABLE 1.11. Catalyst Activity.
Reactor type Comments
Micro-reactor Provides rapid automated screening of several catalysts.
Requires small samples of small catalyst particle.
Compares rate constants, i.e., activity directly.
Allows initial life testing.
Can be used for accelerated aging task.
Eliminates unsuitable formulations.
Continuous stirred tank reactors Use full size catalyst particles.
Carberry—rotating catalyst basket Operates over full range of plant conditions.
