Kim Y.J. (Ed.) Advanced Environmental Monitoring
Подождите немного. Документ загружается.

344 H.N. Kim et al.
1 day after exposure and continued to maintain a high level of expression (up to
tenfold) during the test, although the increasing rate deceased as the exposure
lasted. Likewise, Chg-L expression was strongly induced by 1 µg/L E
2
, demonstrating
the Chg-L is also sensitive to E
2
.
CYP 1A is a representative biomarker for the biotransformation and detoxifica-
tion of xenobiotic compounds. The results in Fig. 26.1 show that CYP 1A expression
tended to decrease as time passed for both concentration of E
2
tested (except for 2
day result at lower concentration). Navas and Segner (2001) found that the ER is
involved in the suppressive action of E
2
on CYP1A expression. Lower CYP1A
production levels can result in deleterious side effects within the fish since the
corresponding protection against harmful xenobiotics, via their metabolism by
CYP 1A, would also be reduced.
HSP 70 is a molecular chaperone that is synthesized under various stressful
conditions. In this study, the HSP 70 mRNA level in the Medaka liver gradually
decreased after exposure to both concentrations of E
2
(Fig. 26.1d). Although these
results indicate that E
2
does not cause any heat shock response within Medaka,
the mechanism by which it influences many of these genes still needs to be
elucidated.
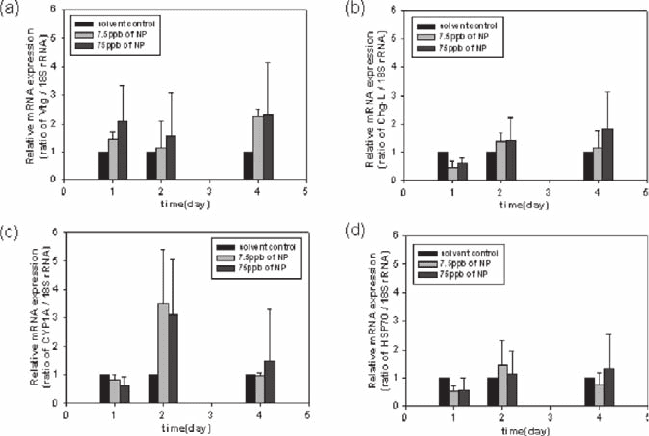
The results after medaka fish were exposed to nonylphenol (NP) are shown in
Fig. 26.2. Whereas the strongest response was seen with the CYP1A gene, the most
sensitive responses were seen from Vtg. Furthermore, Vtg also gave the quickest
Fig. 26.2 Expression patterns of vitellogenin (a), choriogenin-L (b), cytochrome P450 1A (c) and
heat shock protein 70 (d) in the liver of male Medaka after exposure to 7.5 µg/L or 75 µg/L
nonylphenol. (black bar: solvent control, light gray: 7.5 µg/L, dark gray bar: 75 µg/L). All data
were plotted relative to the 18S rRNA expression levels
26 Gene Expression Characteristics in the Japanese Medaka 345
response, with more than a two fold induction after only a one day exposure to
75 µg/L NP. These results demonstrate that NP has an estrogenic effect within
Medaka, albeit a much weaker one than E
2
. Another difference between the
responses to E
2
and NP is a little response from the Chg-L gene with NP, i.e.,
significant responses were seen with E
2
one day after initiating the exposure, but
NP had even decreasing effect on its expression at the first day. Significant increases
in the CYP 1A and HSP 70 expression levels were seen after 2 days when the
Medaka were exposed to 75 µg/L of NP. Induction of CYP 1A implies that NP
caused some form of cellular toxicity in male Medaka since this protein has a
significant role in biotransformation and/or detoxification. In HSP 70 expression,
there is no significant effect on NP exposure.
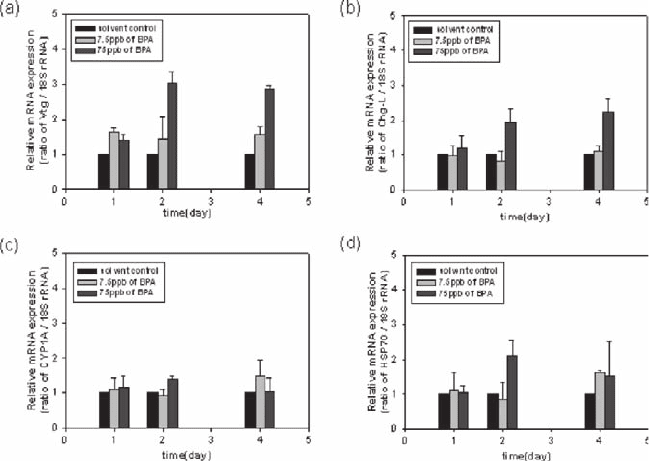
The responses of male Medaka to bisphenol A (BPA) were similar with those
from an exposure to NP. Vtg was induced in a dose-dependent manner, with a
significant induction seen after 2 days for 75 µg/L concentration tested (Fig. 26.3).
As with NP, it appears that the estrogenic effect elicited by BPA is weaker than that
of E
2
. The expression pattern of Chg-L is similar with those of Vtg except but the
less induction level. In CYP 1A expression, there is no significant effect on BPA
exposure. HSP 70 was induced only slightly by the presence of 75 µg/L BPA, but
this induction was maintained, suggesting that high concentrations of BPA cause
some degree of stress.
Fig. 26.3 Expression patterns of vitellogenin (a), choriogenin-L (b), cytochrome P450 1A (c) and
heat shock protein 70 (d) in the liver of male Medaka after exposure to 7.5 µg/L or 75 µg/L of
bisphenol A. (black bar: solvent control, light gray: 7.5 µg/L, dark gray bar: 75 µg/L). All data
were plotted relative to the 18S rRNA expression levels
346 H.N. Kim et al.
In this study, the effects of three different EDCs (17-beta estradiol, nonylphenol
and bisphenol A) were analyzed by measuring the expression levels of specific
biomarkers within Japanese Medaka. The biomarkers used were the genes encoding
for vitellogenin, choriogenin L, cytochrome P450 1A and heat shock protein 70.
Each biomarker differed in its expression pattern depending on the chemical being
tested, and these differences were used to explain the characteristic toxicity
signature of each compound. All three of the chemicals tested are known EDCs that
have estrogenic effects within male Medaka (Yamaguchi et al. 2005). Therefore,
the Vtg and Chg-L genes were selected to investigate and compare the estrogenic
activities of each chemical. The results of this study clearly show that E
2
causes the
strongest estrogenic effect among the compounds tested, while NP and BPA were
similar in their estrogenic activities. Interestingly, exposure to E
2
leads to decreased
CYP1A and HSP 70 expression, a result not seen with NP or BPA. CYP 1A is
specifically responsive to NP, suggesting that NP causes some form of cellular
toxicity that is modulated by CYP 1A. Similarly, HSP 70 genes were induced after
exposure to NP and BPA, indicating that these compounds have some other effects
on the liver cells not only just an estrogenic effect.
In summary, through real time PCR, the expression levels of five genes were
quantified and it was found that each biomarker was differentially expressed
according to the exposure time and concentration of the EDCs (E
2
, NP and BPA)
tested. From these results, the response characteristics of male Japanese Medaka
were successfully investigated and analyzed.
Acknowledgements Authors are grateful to the Laboratory of Freshwater Fish Stocks within
the Bioscience Center, Nagoya University (Chikusa, Nagoya, Japan) for their support in the prepa-
ration of the Japanese Medaka stocks. This work has been financially supported by the ECO
project of the Korean Ministry of Environment, and authors appreciate the support.
References
Birnbaum L.S. and Fenton S.E. (2003), Cancer and developmental exposure to endocrine disrup-
tors, Environ. Health Persp., 111, 389–394.
Bustin S.A. (2002), Absolute quantification of mRNA using real-time reverse transcription
polymerase chain reaction assays, J. Mol. Endocrinol., 25, 169–193.
Flouriot G., Pakdel F., and Valotaire Y. (1996), Transcriptional and post-transcriptional regulation
of rainbow trout estrogen receptor and vitellogenin gene expression, Mol. Cellular Endocrinol.,
124, 173–183.
Gallicchio L., Visvanathan K., Miller S.R., Babus J., Lewis L.M., Zacur H., et al. (2005),
Body mass, estrogen levels, and hot flashes in midlife women, Am. J. Obstet. Gynecol.,
193, 1353–1360.
Janosek J., Hilscherova K., Blaha L., and Holoubek I. (2006), Environmental xenobiotics and
nuclear receptors-interactions, effects and in vitro assessment. Toxicol. In Vitro, 20, 18–37.
Lee C., Na J.G., Lee K.C., and Park K. (2002), Choriogenin mRNA induction in male medaka,
Oryzias latipes as a biomarker of endocrine disruption, Aquat. Toxicol., 61, 233–241.
Lim E.H., Ding J.L., and Lam T.J. (1991), Estradiol-induced vitellogenin gene expression in a
teleost fish, Oreochromis aureus. Gen. Comp. Endocrin., 82, 206–214.
26 Gene Expression Characteristics in the Japanese Medaka 347
Machala M., and Vondracek J. (1998), Estrogenic activity of xenobiotics, Vet. Med.,
43, 311–317.
Mclachlan J.A. (2005), Environmental signaling: What embryos and evolution teach us about
endocrine disrupting chemicals, Endocrine Rev., 22 (3), 319–341.
Murata K., Sugiyama H., Yasumasu S., Iuchi I., Yasumasu I., and Yamagami K. (1997), Cloning
of cDNA and estrogeninduced hepatic gene expression for choriogenin h, a precursor protein
of the fish egg envelope (chorion), Proc. Nat. Acad. Sci. U S A, 94, 2050–2055.
Navas J.M., and Segner H. (2001), Estrogen-mediated suppression of cytochrome p4501a (cyp1a)
expression in rainbow trout hepatocytes: Role of estrogen receptor. Chem.-Biol. Interact.,
138, 285–298.
Yamaguchi A., Ishibashi H., Kohra S., Arizono K., and Tominaga N. (2005), Short-term effects of
endocrine-disrupting chemicals on the expression of estrogen- responsive genes in male
medaka (Oryzias latipes), Aquatic toxicology, 72, 239–249.
Ying G.G., and Kookana R.S. (2002), Endocrine disruption: An Australian perspective. J. Aust.
Water Assoc., 29 (9), 42–45.
Younes M. (1999), Specific issues in health risk assessment of endocrine disrupting chemicals and
international activities, Chemosphere, 39, 1253–1257.

Chapter 27
Optical Detection of Pathogens using
Protein Chip
Jeong-Woo Choi
1,2
and Byung-Keun Oh
1,2
Abstract Optical detection method based protein chips for detection of the
various pathogens such as Escherichia coli O157:H7, Salmonella typhimurium,
Yersinia enterocolitica, and Legionella pneumophila in contaminated environment
were developed. In order to endow the orientation of antibody molecules on
solid surface, protein G was introduced. Gold (Au) surface was modifi ed with
11-mercaptoundecanoic acid (11-MUA) and the protein G was immobilized on
the Au surface. And the spots of different antibodies against pathogens (E. coli
O157:H7, S. typhimurium, Y. enterocolitica, and L. pneumophila) on protein
G of Au surface were arrayed using a microarrayer. The responses of the various
pathogens such as E. coli O157:H7, S. typhimurium, Y. enterocolitica, and
L. pneumophila to the protein chip was investigated by surface plasmon resonance
(SPR), fl uorescence microscopy and imaging ellipsometry (IE). The lowest
detection limit of the fl uorescence based protein chip was 10
2
CFU/mL and the
protein chip using IE could successfully detect the pathogens in concentrations
varying from 10
3
to 10
7
CFU/mL.
Keywords: Fluorescence microscopy, imaging ellipsometry, protein chip,
pathogen, protein G, surface plasmon resonance
27.1 Introduction
As the sequencing of the human genome project (HRP) has been finalized,
biological research is entering a new era in which experimental focus will shift
from identifying novel genes to determining the function of gene products
1
Department of Chemical and Biomolecular Engineering, Sogang University, #1
Shinsu-dong, Mapo-gu, Seoul 121–742, Korea; Tel: (+82) 2–705–8480, Fax: (+82)
2–3273–0331
2
Interdisciplinary Program of Integrated Biotechnology, Sogang University, #1
Shinsu-dong, Mapo-gu, Seoul 121–742, Korea
348
Y.J. Kim and U. Platt (eds.), Advanced Environmental Monitoring,
348–362.
© Springer 2008
27 Optical Detection of Pathogens using Protein Chip 349
(Pandey and Mann 2000). Rising to this challenge, several technologies have
emerged that aim to characterize genes and/or proteins collectively rather than
individually. Protein chip technology holds significant promise as a high-throughput
platform for the structural and functional characterization of the components of
the proteomes of humans, plants, animals, and microbes, etc. (Kelvin 2001;
Wilson and Nock 2001). Protein chip is an array in which each protein occupies
a defined spot on the chip. Such devices would be employed for highly parallel
studies of the activities of native proteins and serve as an analytical tool
somewhat analogous to DNA chips in the sense that it would be capable of
monitoring protein levels in a given biological sample in a massively parallel
fashion. Given the huge potential market for such devices, industrial interest in
protein chip is very high. In order to develop the protein chips as the ideal
proteomics-based analytical tool, it needs to be efficiently resolved the problems
in the fabrication of protein chips, such as the ligand production, the proteins
immobilization onto solid surface, and the monitoring of the proteins binding to
the chips, etc. (Cahill 2001; Kodadek 2001).
The types of surfaces to which proteins can be immobilized fall into two
categories. The first and simplest type of immobilization is physically adsorbed
onto surfaces by van der Waals, hydrophobic and hydrogen-bonding interactions.
The advantage of this type of immobilization is that it is very simple to perform
due to not requirement of any modification of the protein. The disadvantage is
that most of the immobilized protein can be inactivated due to denaturation and
steric occlusion (Butler 1992). A preferred method relies on one or a small
number of strong bonds between the protein and surface, leaving the protein
largely unaltered, except in the vicinity of the contact point. For examples, it
would be included the covalent attachment of protein, immobilization of bioti-
nylated proteins onto streptavidin-coated surface, and immobilization of His-
tagged proteins onto Ni
2+
-chelating surface (Arenkov et al. 2000; Ruiz-Taylor et
al. 2001; Zhu et al. 2001). However, due to this common methods for immo-
bilizing proteins through (or biotin-based) interactions is by randomly conjugat-
ing lysine residues on proteins to amine-reactive surfaces (or biotinylation
reagents), in order to construct the protein chips with high performance, it needs
to be develop the immobilization techniques of protein in an oriented fashion
(Choi et al. 2001, 2004; Vijayendran and Leckband 2001; Wilson et al. 2002).
For the construction of a well-defined antibody surface, protein G, a cell wall
protein found in most species of Streptococci, can be used as the binding material.
Since protein G has a specific interaction with the F
c
portion of Immunoglobulin
G (IgG) (Boyle and Reis 1987), the paratope of IgG can face the opposite side
of the protein G-immobilized solid support. As a result, protein G-mediated
antibody immobilization can lead to a highly efficient immunoreaction
(Kretschmann E. 1971; Bae et al. 2004).
In protein chip, the binding property of antigen to antibody is commonly
monitored by optical detection methods. One of them is fluorescence (Angenendt
et al. 2003; Choi et al. 2006; Oh et al. 2007). The assay utilizes two antibodies that
simultaneously bind the same antigen: one is immobilized onto a solid surface, and the
350 J.-W. Choi and B.-K. Oh
other is fluorescently labeled that can produce a fluorescent, luminescent, or colored
product. Although this approach has some problems; the chemical heterogeneity
of proteins makes this hopeless as a strategy for doing quantitative work because
some proteins will label far more efficiently than others, and chemically labeling
of proteins results in changes of their surface characteristics greatly, and a lot of
labeled antibodies for all proteins is required (Kodadek T. 2001), fluorescence
based detection method must be easily available in protein chip.
Otherwise there are some optical techniques with enough sensitivity to detect
the binding of antigens to antibodies without the requirement of labeling process
and secondary antibody labeled with a dye. Surface plasmon resonance (SPR) and
imaging ellipsometry (IE) sensors have been developed to measure the binding of
analytes to sensor surface, which are capable of directly detecting analytes in
complex biological media with high sensitivity, with a short detection time, and
with simplicity (Darren et al. 1998; Sakai et al. 1998; Bae et al. 2004; Oh et al.
2005).The SPR technique, an optical method based on the attenuation of surface
plasmon generated between a metal surface and a dielectric layer, has matured to
become a versatile detection tool for the study of the kinetics of receptor-ligand
interaction, the adsorption of biopolymer on solid surface, peptide-antibody binding,
and protein-protein interaction. IE technique is based on ellipsometry and the other
optical technique involves measuring the change of the polarization state of an
elliptically polarized beam reflected from thin film. It is sensitive enough to detect
the adsorption of a molecular monolayer on a solid surface, such as a silicon wafer
or gold surface.
Bacterial pathogens existing in contaminated environment such as E. coli O157:
H7, Salmonella spp., Yersinia spp. and Legionella spp. pose a significant threat
to human, animal, and agricultural health. Detection of pathogens existing in
contaminated environment, therefore, is very important for public health protection
(Black et al. 1978; Hussong et al. 1987; Cowden and Christie 1997; Pathirana et al.
2000; Wong et al. 2002). Consequently, considerable effort has been devoted to
developing rapid, sensitive, and specific assays for these organisms. However,
conventional microbiological culture methods used for the detection of micro-
organism are labour-intensive and requires several days to obtain results and may
be unsatisfactory to respond in a timely manner in cases of contamination. Many
immunoassay techniques are widely attempted for the detection of bacteria, but
they are usually expensive and require time-consuming and complex sample
pretreatment procedures. For example, enzyme-linked immunosorbent assay
(ELISA) is the most frequently used immunochemical approach to detect
pathogens with detection limits ranging from 10
4
to 10
6
colony-forming units
(CFU) per mL requiring enrichment usually for 16–24 h (De Boer and Beumer
1998; Kim et al. 1999). Therefore, alternative methods to simultaneously detect
pathogens in contaminated environment with high sensitivity, with a short detection
time, and with simplicity may be need.
In this study, the objective is to optically detect the various pathogens such as
Escherichia coli O157:H7, Salmonella typhimurium, Yersinia enterocolitica,
and Legionella pneumophila using protein chip. In order to endow the orientation
27 Optical Detection of Pathogens using Protein Chip 351
of antibody molecules on solid surface, protein G was introduced. The different
antibodies against pathogens (E. coli O157:H7, S. typhimurium, Y. enterocolitica,
and L. pneumophila) on self-assembled protein G were selectively arrayed using a
microarrayer. The responses of the each pathogen to the protein chip were investigated
by SPR, fluorescence microscopy, and IE.
27.2 Experimental
27.2.1 Materials
Protein G (M.W. 22,600 Daltons) was purchased from Prozyme Inc. (USA).
S. typhimurium (KCCM 11806) was kindly donated from the Korean Culture
Center of Microorganisms (Korea). E. coli O157:H7 (ATCC 43895) and Y. enterocolitica
(ATCC 700823) was kindly donated from the American Type Culture Collection
(USA). L. pneumophila (ATCC 33154) was kindly offered from National
Institute of Health in Korea. Monoclonal antibody (Mab) against S. typhimu-
rium and Mab against L. pneumophila – fluorescein isothiocyanate (FITC) conjugate
were obtained from Biogenesis, Ltd. (USA). Mab against E. coli O157:H7, Mab
against E. coli O157:H7 – FITC conjugate, and Mab against L. pneumophila were
obtained from Fitzgerald Industries International, Inc. (USA). Mab against
Y. enterocolitica, Mab against Y. enterocolitica – FITC conjugate, and Mab
against Salmonella spp. conjugate were obtained from Biodesign International,
Inc. (USA). Other chemicals used in this study were obtained commercially as
the reagent grade.
27.2.2 Immobilization of Antibody
BK 7 glass plate (18 mm × 18 mm, Superior, Germany) was used as the solid
support and Au was sputtered to the BK 7 glass surface. Before sputtering Au,
chromium (Cr) was sputtered on the glass slide to promote the adhesion of Au.
The Au and the Cr film had a thickness of 43 ± 1 nm and 2 nm, respectively. The
Au surface was cleaned using pirahna solution (30 vol.% H
2
O
2
and 70 vol.%
H
2
SO
4
) at 60°C for 5 min, and then rinsed with ethanol and deionized water. The
self-assembled monolayer of 11-mercaptoundecanoic acid (11-MUA) on the Au
surface was fabricated by submerging the prepared Au substrate into a glycerol/
ethanol (1:1, v/v) solution containing 150 mM of 11-MUA for at least 12 h (Yam
et al. 2001). For chemical binding between the 11-MUA adsorbed on the Au
substrate and the free amine from the protein G, the carboxyl group in 11-MUA
was activated by submerging the Au substrate modified with 11-MUA into a solution
of 10% 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC)
in water/ethanol (10/1, v/v) for 2 h at room temperature. The self-assembled
352 J.-W. Choi and B.-K. Oh
protein G layer was fabricated by the incubation of the activated Au substrate in a
solution of protein G in 10 mM phosphate buffer (PBS, pH 7.4) containing
0.14 mol/L NaCl and 0.02% (w/v) thimerosal (PBS) at room temperature for 2 h
Before the immobilization of the antibody, the protein G layer by self-assembly
technique on the Au substrate was blocked by inactivating the residual carboxyl
group of 11-MUA with 1 M of ethanolamine. To immobilize the Mab, the protein
G layer by self-assembly technique was immersed in a solution containing antibodies
(50 pmol/mL Mabs) in a PBS buffer because the antibody surface loading on
self-assembled protein G layer started to be saturated at 50 pmol/mL. After 4 h of
incubation at 4°C, the surface was rinsed with a PBS buffer. In order to provide
antigen access to the binding site of antibody by separation of antibody molecules
clustered around preferred points on the surface or around other antibody molecules,
Tween 20 was used.
27.2.3 Preparation of Protein Chip Based on SPR
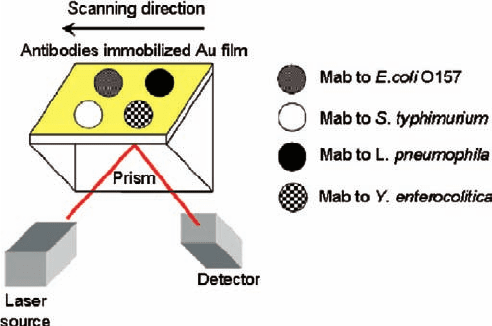
The schematic illustration of protein chip system based on SPR was shown in
Fig. 27.1. The metal coating and substrate cleaning, and the immobilization of
biomolecules onto Au patterned SPR surface was performed in the same way as
in above mentioned procedure. The pattern size was dia. 3 mm. The bimolecular
interactions of protein chip were monitored using a SPR spectroscope (Multiskop,
Optrel GbR, Germany) (Harke et al. 1997). A He-Ne laser was used as a light
source to make a monochromatic light with a wavelength of 632.8 nm. The
p-polarized light beam by the polarizer is used as a reference and the intensity of
Fig. 27.1 The schematic illustration of protein chip system based on SPR
27 Optical Detection of Pathogens using Protein Chip 353
the reflected beam is measured by photo multiplier tube (PMT) sensor. A 90°
glass prism (BK 7, n = 1.5168) is used as a Kretschmann ATR coupler (Kretschmann
1971). The plane face of the 90° glass prism was coupled to a BK 7 glass slide
via index matching fluid. The resolution of the angle reading of the goniometer
was 0.001°.
27.2.4 Preparation of Protein Chip Based
on Imaging Ellipsometry
A substrate was prepared by DC magnetron sputtering of Au on a P-type Si
wafer. The metal coating and substrate cleaning, and the immobilization of
biomolecules were performed in the similar way as in above mentioned proce-
dure. The Au and Cr films had thicknesses of 150 and 5 nm, respectively. The
protein G solution was spotted onto 11-MUA modified Au surface using a
microarrayer (NanoPlotter model 1.2, GeSiM mbH, Groβerkmannsdorf, Germany).
The spotted amount per spot was 0.4 nL of a solution of 0.1 mg/mL protein G in
a mixed solution of 10 mM PBS buffer and 10 vol% glycerol. The spotted sub-
strate was incubated in a humid chamber at 4°C for at least 24 h, taking into
consideration the diffusivity delay of the protein molecules, which are hindered
from entering into the surface due to the viscosity of glycerol. After the incuba-
tion period, the chip was washed with PBS buffer for 20–30 min. Before the
immobilization of the Mab, the residue carboxyl groups of 11-MUA on the chip
were inactivated by blocking them with 3 wt% bovine serum albumin (BSA). A
solution containing the Mab in PBS buffer was applied to the blocked chip. After
being incubated at 4°C for 3 h, the substrate was washed with PBS buffer
containing 0.1% Tween 20.
27.2.5 Preparation of Protein Chip Based on Fluorescence Image
A BK 7 type cover glass plate was used as the solid support. The metal coating
and substrate cleaning, and the immobilization of biomolecules were performed in
the similar way as in above mentioned procedure. The protein G was arrayed on
11-MUA modified Au surface using a microarrayer. And then the antibody
molecules such as Mab against E. coli O157:H7, Mab against S. typhimurium,
Mab against Y. enterocolitica, and Mab against L. pneumophila were spotted on
the protein G by using the microarrayer. After incubation at 4°C for at least 24 h,
the surface was washed with PBS buffer containing 0.1% Tween 20. The residue
carboxyl groups of 11-MUA on protein chip were inactivated by blocking them
with 3 wt % BSA.
