Heywood J.B. Internal Combustion Engines Fundamentals
Подождите немного. Документ загружается.

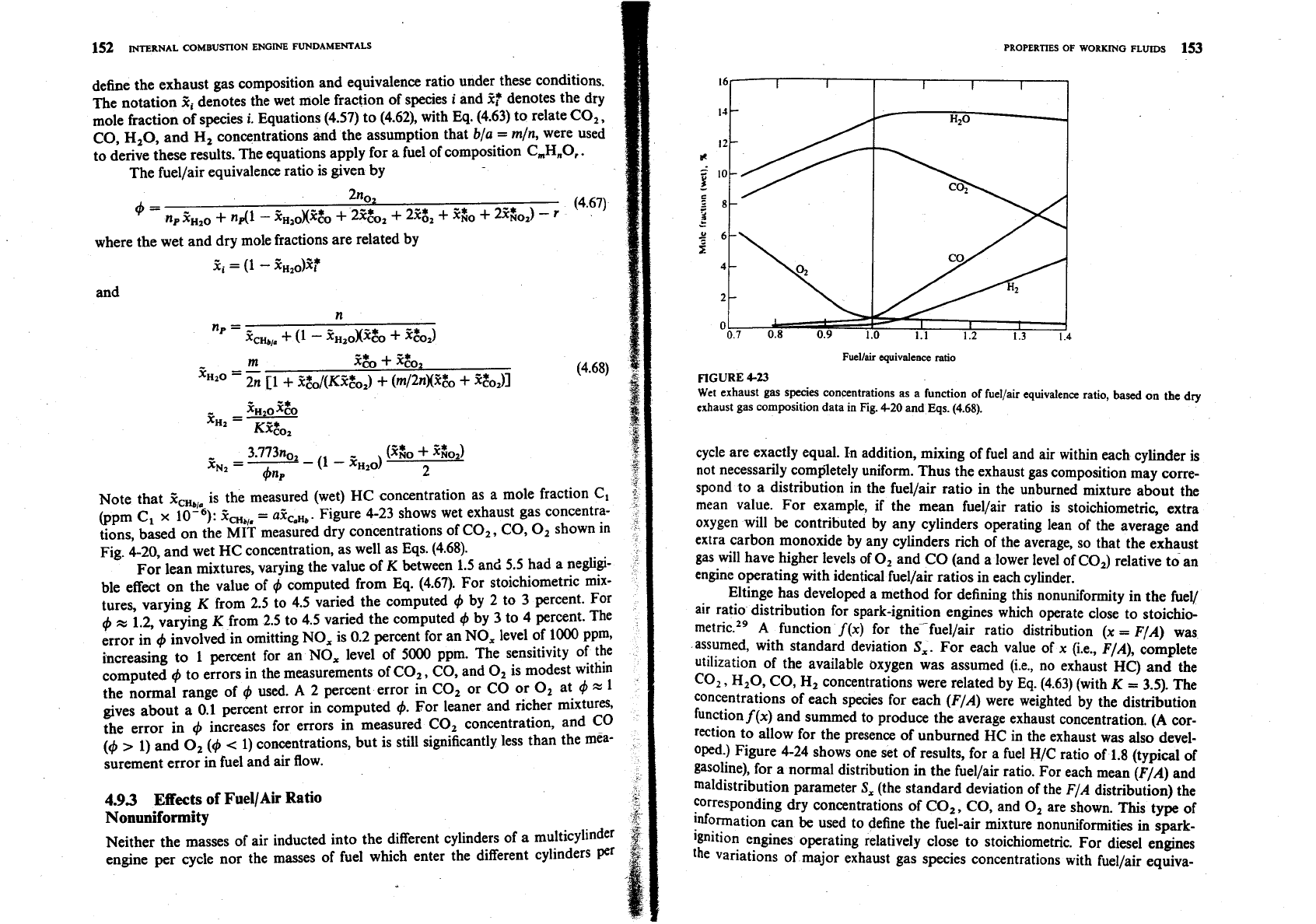
define the exhaust gas composition and equivalence ratio under these conditions.
The notation
jti
denotes the wet mole fraction of species
i
and
5:
denotes the dry
mole fraction of species
i.
Equations (4.57) to (4.62), with Eq. (4.63) to relate CO,,
CO, H,O, and H, concentrations and the assumption that
b/a
=
m/n, were used
to derive these results. The equations apply for
a
fuel of composition C,H,O,
.
The fuellair equivalence ratio is given by
where the wet and dry mole fractions are related by
jti
=
(1
-
ZHZO)q'
and
Note that
X,,,,,
is the measured (wet) HC concentration as a mole fraction C,
@pm C,
x
ZCHb,,
=
aZCaHb. Figure 4-23 shows wet exhaust gas concentra-
tions, based on the
MIT
measured dry concentrations of CO,
,
CO,
0,
shown in
Fig. 4-20, and wet HC concentration, as well as Eqs. (4.68).
For lean mixtures, varying the value of
K
between 1.5 and 5.5 had a neglgi-
ble effect on the value of
4
computed from Eq. (4.67). For stoichiometric mix-
tures, varying
K from 2.5 to 4.5 varied the computed
4
by 2 to
3
percent. For
4
z
1.2, varying K from 2.5 to 4.5 varied the computed
4
by
3
to 4 percent. The
error in
4
involved in omitting NO, is 0.2 percent for an NO, level of 1000 ppm,
increasing to 1 percent for an NO, level of 5000 ppm. The sensitivity of the
computed
4
to errors in the measurements of CO,
,
CO, and
0,
is modest within
the normal range of
4
used.
A
2 percent error in CO, or CO or
0,
at
4
x
1
gives about a 0.1 percent error in computed
4.
For leaner and richer mixtures,
the error in
9 increases for errors in measured CO, concentration, and CO
(4
>
1) and
0,
(4
<
1)
concentrations, but is still significantly less than the mea-
surement error in fuel and air flow.
4.93 Effects
of
Fuel/
Air
Ratio
Nonuniforrnity
Neither the masses of air inducted into the different cylinders of a multicylinder
engine per cycle nor the masses of fuel which enter the different cylinders Per
Fuellair
equivalence
ratio
FIGURE
4-23
Wet
exhaust gas species concentrations as a function
of
fuel/air equivalence ratio,
based on
the
dry
exhaust gas composition data in Fig.
4-20
and Eqs.
(4.68).
cycle are exactly equal. In addition, mixing of fuel and air within each cylinder is
not necessarily completely uniform. Thus the exhaust gas composition may corre-
spond to a distribution in the fuellair ratio in the unburned mixture about the
mean value. For example, if the mean
fuellair ratio is stoichiometric, extra
oxygen will be contributed by any cylinders operating lean of the average and
extra carbon monoxide by any cylinders rich of the average, so that the exhaust
gas will have higher levels of
0,
and CO (and a lower level of CO,) relative to an
engine operating with identical fuellair ratios in each cylinder.
Eltinge has developed a method for defining this nonuniformity in the fuel/
air ratio distribution for spark-ignition engines which operate close to
stoichio-
metric.29
A
function
f(x)
for theafuel/air ratio distribution
(x
=
F/A) was
assumed, with standard deviation
S,.
For each value of
x
(i.e., FIA), complete
utilization of the available bxygen was assumed (i.e., no exhaust HC) and the
CO,, H,O, CO, H, concentrations were related by Eq. (4.63) (with
K
=
3.5). The
concentrations of each species for each (FIA) were weighted by the distribution
function
f
(x)
and summed to produce the average exhaust concentration.
(A
cor-
rection to allow for the presence of unburned HC in the exhaust was also devel-
oped.) Figure 4-24 shows one set of results, for a fuel H/C ratio of 1.8 (typical of
gasoline), for a normal distribution in the fuellair ratio. For each mean (FIA) and
maldistribution parameter
S,
(the standard deviation of the FIA distribution) the
corresponding dry concentrations of CO,, CO, and
0,
are shown. This
type
of
information can
be
used to define the fuel-air mixture nonuniformities
in
spark-
ignition engines operating relatively close to stoichiometric. For diesel engines
'he variations of major exhaust gas species concentrations with fuellair equiva-
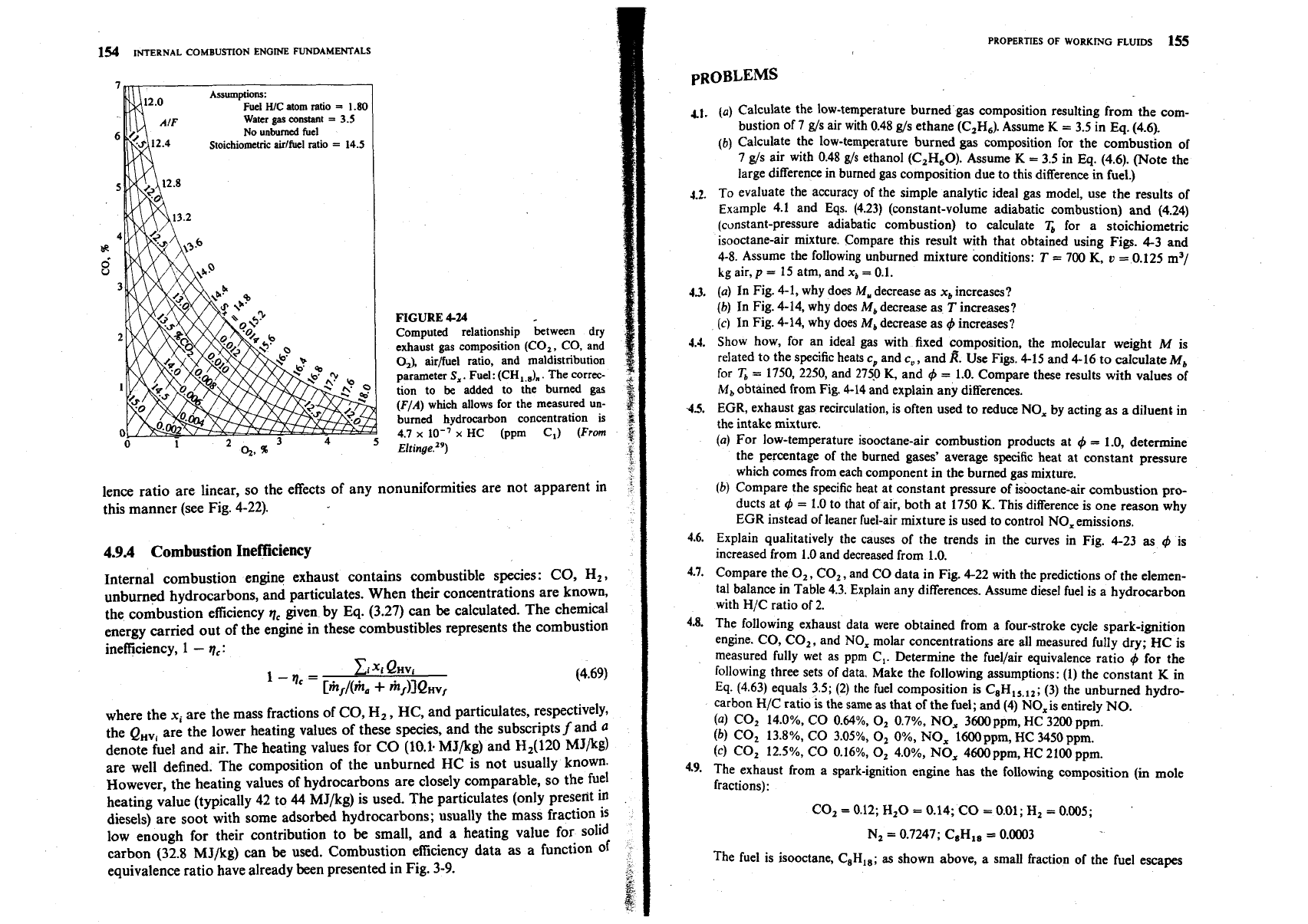
154
INTERNAL
COMBUSTION
ENGINE
FUNDAMENTALS
Assumptions:
Fuel
HIC
atom
ratio
=
1.80
Water
gas
constant
=
3.5
No
unburned
fuel
Stoichiometric
airlfuel
ratio
=
14.5
FIGURE
4-24
Computed relationship between dry
exhaust gas composition (CO,, CO, and
O,), airjfuel ratio, and maldistribution
parameter
S,.
Fuel: (CH,.,),
.
The correc-
tion to
be
added to the burned gar
(FIA)
which allows for the measured
un-
burned hvdrocarbon concentration is
5
4.7
x
lo-;
x
HC (ppm
C,)
(From
$
~Itinge.'')
k
lence ratio are linear, so the effects
of
any nonuniformities are not apparent in
this manner (see Fig. 4-22).
4.9.4
Combustion Inefficiency
Internal combustion engine exhaust contains combustible species: CO, Hz,
unburned hydrocarbons, and particulates. When their concentrations are known,
the combustion efficiency
rt,
given by Eq. (3.27) can
be
calculated. The chemical
energy carried out of the engine in these combustibles represents the combustion
inefficiency,
1
-
q,
:
where the
xi
are the mass fractions
of
CO,
H,
,
HC, and particulates, respectively,
the
Q,,,
are the lower heating values of these species, and the subscripts
f
and
denote fuel and air. The heating values for CO (10.1. MJ/kg) and Hz(120 MJFg)
are well defined. The composition of the unburned HC is not usually known.
However, the heating values of hydrocarbons are closely comparable, so the fuel
heating value (typically 42 to
44
MJ/kg) is used. The particulates (only present in
diesels) are soot with some adsorbed hydrocarbons; usually the mass fraction is
low enough for their contribution to be small, and
a
heating value for solid
carbon (32.8 MJ/kg) can
be
used. Combustion efficiency data as a function
of
-
equivalence ratio have already been presented in Fig. 3-9.
fl
f
PROPERTIES
OF
WORKING
FLUIDS
155
PROBLEMS
(a) Calculate the low-temperature burned gas composition resulting from the com-
bustion of
7
g/s air with 0.48 g/s ethane (C,H,). Assume
K
=
3.5 in
Eq.
(4.6).
(b)
Calculate the low-temperature burned gas composition for the combustion of
7 g/s air with 0.48 g/s ethanol (C,H,O). Assume
K
=
3.5
in Eq. (4.6). (Note the
large difference in burned gas composition due to this difference in fuel.)
4.2.
TO
evaluate the accuracy of the simple analytic ideal gas model, use the results of
Example 4.1 and Eqs. (4.23) (constant-volume adiabatic combustion) and (4.24)
(constant-pressure adiabatic combustion) to calculate
T,
for a stoichiometric
isooctane-air mixture. Compare this result with that obtained using Figs. 4-3 and
4-8. Assume the following unburned mixture conditions:
T
=
700
K,
v
=
0.125 m3/
kg
air,
p
=
15 atm, and
xb
=
0.1.
43.
(a) In Fig. 4-1, why does
M,
decrease as
x,
increases?
(b) In Fig. 4-14, why does
Mb
decrease as
T
increases?
(c)
In Fig. 4-14, why does
Mb
decrease as
4
increases?
4.4.
Show how, for an ideal gas with fixed composition, the molecular weight
M
is
related to the specific heats
c,
and c,, and
J?.
Use Figs. 4-15 and 4-16 to calculate
Mb
for
&
=
1750, 2250, and 2750
K,
and
4
=
1.0. Compare these results with values of
M,
obtained from Fig. 4-14 and explain any differences.
45.
EGR,
exhaust gas recirculation, is often used to reduce NO, by acting
as
a
diluent in
the intake mixture.
(a) For low-temperature isooctane-air combustion products at
4
=
1.0, determine
the percentage of the burned gases' average specific heat at constant pressure
which comes from each component in the burned gas mixture.
(b) Compare the specific heat at constant pressure of isooctane-air combustion pro-
ducts at
4
=
1.0 to that of air, both at 1750
K.
This difference is one reason why
EGR
instead of leaner fuel-air mixture is used to control NO, emissions.
4.6.
Explain qualitatively the causes of the trends in the curves in Fig. 4-23
as
4
is
increased from 1.0 and decreased from 1.0.
4.7.
Compare the
0,
,
CO,
,
and CO data in Fig. 4-22 with the predictions of the elemen-
tal balance in Table 4.3. Explain any differences. Assume diesel fuel is a hydrocarbon
with H/C ratio of 2.
4.8.
The following exhaust data were obtained from a four-stroke cycle spark-ignition
engine. CO, CO,, and
NO,
molar concentrations are all measured fully dry; HC is
measured fully wet as ppm C,. Determine the fuellair equivalence ratio
4
for the
following three sets of data. Make the following assumptions: (1) the constant
K
in
Eq. (4.63) equals 3.5; (2) the fuel composition is C,H,,,,,; (3) the unburned hydro-
carbon H/C ratio is the same as that of the fuel; and (4) N0,is entirely NO.
(a) CO, 14.0%, CO OH%,
0,
0.7%, NO, 3690ppm, HC 3200ppm.
(b)
CO, 13.8%, CO 3.05%,
0,
Ox, NO, 1600ppm, HC 34% ppm.
(c) CO, 12.5%, CO 0.16%,
0,
4.0%, NO, 4600ppm, HC 2100 ppm.
4.9.
The exhaust from a spark-ignition engine has the following composition (in mole
fractions):
The
fuel is isooctane, C,H,,; as shown above, a small fraction of the fuel escapes
156
INTERNAL
COMBUSTION ENGINE
FUNDAMENTALS
from the cylinder unburned. The lower heating value of isooctane is 44.4 MJ/kg, of
carbon monoxide is 10.1 MJ/kg, and of hydrogen is 120 MJ/kg. The atomic weights
of the elements are: C
=
12.0
=
16, H
=
1.
N
=
14.
(a) Calculate the combustion inenlciency in the engine; i.e., the percentage of the
entering fuel enthalpy which is not fully released in the combustion process and
leaves the engine in the exhaust gases (for this problem, the exhaust can
be
assumed to be at room temperature).
(b) What fraction of this inefficiency is due to the unburned fuel emissions?
.
.
4.10. A %liter displacement four-cylinder engine, operating at 2000 rev/min and 30
percent of its maximum power at that speed, has the following exhaust composition
(in percent by volume or mole percent):
C02, 11%; H20, 11.5%; CO, 0.5%;
Hz,
0%; O,, 2%; unburned hydrocarbons
(expressed as CH,, i.e., with a molecular weight of 14), 0.5%; N2,74.5%
The fuel is (CH,), with a heating value of
44
MJ/kg. The atomic weights of the
elements are
C
=
12,
H
=
1,0
=
16,
N
=
14. The heating values of CO and
HC
are
10 and
44
MJ/kg, respectively.
(a) Is the engine a diesel or spark-ignition engine? Is there enough oxygen in the
exhaust to burn the fuel completely? Briefly explain.
(b) Calculate the fraction of the input fuel energy
(m,Q,,)
which exits the engine
unburned as (1) CO and (2) unburned HC.
(c)
An inventor claims a combustion efficiency of 100 percent
can
be
achieved. What
percentage improvement in engine specific fuel consumption would result?
4.11. A gasoline engine operates steadily on a mixture of isooctane and air. The air and
fuel enter the engine at 25OC. The fuel consumption is 3.0 as. The output of the
engine is 50 kW. The temperature of the combustion products in the exhaust mani-
fold is
660
K. At this temperature, an analysis of the combustion products yields the
following values (on a dry volumetric basis):
CO,, 11.4%;
02,
1.6%; CO, 2.9%; N2, 84.1%
(a) Find the composition in moles (number of moles per mole of isooctane) of the
. .
reactants and the reaction products.
(b) Determine the heat-transfer rate from the working fluid as the working fluid
passes through the engine.
Constants for the calculations:
Enthalpy of formation,
Sensible
enthalpy at
660
K,
kJ/kmol kJ/kmol
4.12 A direct-injection four-stroke cycle diesel engine is used to provide power for
pumping water. The engine operates at its maximum rated power at 2000 rev@&
PROPERTIES
OF
WORKING
FLUIDS
157
with an equivalence ratio of 0.8 and an air flow of 0.5 kas. The gross indicated fuel
conversion efficiency is 45 percent, and the heat losses from the working fluid to the
engine coolant and elsewhere within the engine are 280
kW.
Diesel fuel has a heating
value of 42 MJ/kg and stoichiometric fuel/air ratio of 0.067. Fuel and air enter the
engine at ambient conditions. The mechanical efficiency of the diesel engine is 85
percent.
(a)
Calculate the rated brake power of the engine, the average sensible enthalpy of
the exhaust gases as they leave the engine, and the average exhaust gas tem-
perature.
(b) Since the exhaust gas temperature is significantly above ambient, the advantages
of using the diesel exhaust gas stream to heat the boiler of a
Rankine cycle (see
sketch) and generate additional power are to be explored. If the exhaust gases
leave
the Rankine-cycle system boiler at 400
K
and 30 percent of the heat trans-
ferred from the exhaust gas stream in the boiler is converted to power at the
Rankine-cycle power plant drive shaft, calculate the additional power obtained
and the brake fuel conversion efficiency of the combined cycle system (diesel plus
Rankine cycle).
4.13.
A
diesel engine has a compression ratio of 22
:
1. The conditions in the cylinder at
the start of compression are
p
=
101.3 kPa and
T
=
325
K.
Calculate the pressure
and temperature at the end of compression, assuming the compression process is
isentropic:
r
Diesel
engine
(a) ~ssume the cylinder contains an ideal gas with
y
=
1.4 and
R
=
287
J/kg
.
K.
-
Hot
Rankine cycle boiler cooled
diesel diesel
exhaust exhaust
400
K-
I
(b)
Assume the cylinder contains air which may
be
regarded as a semiperfect gas (use
the gas tables).
(c)
Compare the work of compression in (a) and (b) above.
In practice, heat losses reduce the final compression temperature by 100 K. For a
diesel engine operating at
an
equivalence ratio_ of 0.75 (full load):
(6)
Calculate the ratio of heat loss during compression to the fuel energy added per
cycle.
7
t
Rankine (liquid)
cycle fluid
(vapor)
FIGURE
P4-12
4.14.
While the geometric compression ratio of an engine is
VJV,,,,
the actual compres-
sion process starts somewhere between bottom-center and when the inlet valve
closes, and it is conditions at time of spark (for an SI engine) or fuel injection (for a
CI engine) that determine ignition. At low engine speed, compression starts about
the time when the inlet valve closes. With this
assuplption, for the diesel engine of
Prob. 4.13, calculate the air pressure and temperature at the start of injection. The
inlet valve closes at
30' after BC; injection commences 15" before TC. Use the gas
tables. Compare your answers with those of Prob. 4.13(b).
158
INTERNAL
COMBUSTION
ENGINE
FUNDAMENTALS
4.15. Use an equilibrium computer code (which calculates the composition and properties
of chemically reacting gas mixtures in equilibrium) to calculate the data you need for
the following graphs:
(a) Values of c,,
y,
molecular weight, and
gas
composition (mole fractions of
N,,
CO,, H20. CO, Hz, O,, OH, 0, H, and NO)
as
a function of the equivalence
ratio (4
=
0.2 to 1.4) for products of combustion of isooctane (C,H,,) with air at
p
=
40
atm and
T
=
2500
K.
Put all species concentrations on the same graph.
Use a log scale for the composition axis.
(b)
Unburned mixture consisting of isooctane vapor and
air
at 700 K and 20 atm is
burned fist at constant pressure and then at constant volume.
(1) Calculate the enthalpy and internal energy of isooctane vapor at
700
K
in
cal/gmol; also calculate the volume per unit mass of mixture (cm3/g) for
4
=
0.2,0.4,0.6,0.8,
1.0,
1.2, 1.4.
(2) Use these data and the equilibrium program to calculate the temperature
attained after combustion at constant pressure, and temperature and pressure
attained after combustion at constant volume. Plot these temperatures and
pressures against the equivalence ratio
4.
Thermodynamic
data
for
isooctane vapor
T,
K
Z,,
cal/mol-
K
%
-
&,,
,
kd/mol
&-;,
kd/mol
298 45.14
0.00
-
53.57
700 85.66
27.02
-
62.79
4.16.
A
heavy wall bomb with a volume of 1000 cm3 contains a mixture of isooctane with
the stoichiometric air requirement at
p
=
101.3 kPa and
T
=
25•‹C. The mixture is
then ignited with a spark. Find the pressure and temperature of the equilibrium
combustion products just after combustion is complete
(i.e., before heat losses to the
wall are significant).
Assume the burned gases are uniform.
4.17.
A
gas engine, running on a gaseous mixture of butane,
C,H,,,
and air has the
following conditions in the cylinder prior to constant-volume adiabatic combustion:
pressure, 6.48
x
loS
N/m2; temperature,
600
K.
The charge composition is air plus
50 percent of the stoichiometric quantity of butane fuel. Calculate the pressure and
temperature at the end of combustion using the data given below.
For
air
T,
K
Q
Jlpof
For
butane
T,
K
c,
JIgmol
PROPERTIES
OF
WORKING
FLUIDS
159
Internal energy of combustion of butane at 298
K
is
Air
=
-2.659 MJ/gmol.
Extract from gas tables for products of combustion for 50 percent stoichiometric
fuel
:
REFERENCES
1.
Komiyama, K., and Heywood,
J.
B.: "Predicting NO, Emissions and the Effects of Exhaust
Gas
Recirculation in Spark-Ignition Engines;" SAE paper 730475,
SAE
Trans.,
vol. 82, 1973.
2.
Danleli,
G.,
Ferguson, C., Heywood, J., and Keck, J. "Predicting the Emissions and Performance
Characteristics of a Wankel Engine," SAE paper 740186,
SAE
Trans.,
vol. 83,1974.
1.
Hottel,
H.
C., Williams,
G.
C., and Satterfield, C. N.:
Thermodynamic Charts for Combustion
Processes,
John Wiey, 1949.
See
also charts in C. F. Taylor,
The Internal Combustion Engine in
Theory
and
Practice.
vol. 1, MIT Press, 1960.
4.
Newhall, H.
K..
and Starkman, E. S.: "Thermodynamic Properties of Octane and Air for Engine
Performance Calculations," in
Digital Calculations of Engine Cycles, Progress in Technology,
vol.
TP-7, pp. 38-48, SAE, 1964.
5
Starkman, E. S., and Newhall,
H. K.:
"Thamodynamic Properties of Methane and Air, and
Propane and Air for Engine Performance Calculations," SAE paper 670466,
SAE
Trans.,
vol. 76,
1967.
6.
Keenan, J. H., Chao, J., and Kaye, J.:
Gas
Tables,
2d ed., John Wiey, 1983.
7. Reynolds,
W.
C.:
ThermodynMlie Properties
in
SI; Graphs, Tables, and Computational Equations
/or Forty Substances,
Department of Mechanical Engineering, Stanford University, 1979.
8.
JANAF
Thmnochemical Tables,
2d
ad.,
NSRDS-NB537, U.S. National Bureau
of
Standards,
June 1971.
9. Gordon, S., and McBride, B. J.: "Computer Program for the Calculation of Complex Chemical
Equilibrium Composition, Rocket Pcrformanoe, Incident and Reflected Shocks, and Chapman-
Jouguet Detonations;" NASA publication SP-273,1971 (NTH number N71-37775).
10.
Svehla,
R.
A., and McBride, B.
J.:
"Fortran
IV
Computer Program for Calculation of Thermody-
namic and Transport Properties of Complex Chemical Systems," NASA technical note TND-
7056.1973 (NTIS number N73-15954).
I
I
Fremont,
H.
A.,
et al.: Properties of Combustion Gases,
General Electric Company, Cincinnati,
Ohio, 1955.
11 Banes, B., McIntyrc, R. W., and Sims, J.
A:
Properties of Air
and
Combustion Products with
Kerosene
and
Hydrogen Fuels,
vols. I-XIII, Propulsion and Energetics Panel, Advisory Group for
Aerospace Research and Development (AGARD), NATO, published by Bristol Siddeley Engines
Ltd., Filton, Bristol, England, 1967.
13.
Hires, S. D., Ekchian, A., Heywood, J. B, Tabaczynski, R. J., and Wall, J. C.: "Performana and
NO, Emissions ModelSig of a Jet Ignition Rechamber Stratified Charge Engine," SAE paper
760161,
SAE
Trans.,
vol. 85,1976.
Id.
LoRusso, J.
A:
"Combustion and Emissions Characteristics of Methanol, Methanol-Water, and
Gasoline-Methanol Blends
in
a Spark Ignition Engine," S. M. Thesis, Department of Mechanical
,
Engineering, MIT, May 1976.
IJ.
BY.
A.,
Kempinski, B, and Rife, J.
M.:
"Knock
in Spark-Ignition Engines," SAE paper 810147,
1981.

16.
Rossini.
F.
D..
Pitzer, K. S., Arnett, R. L., Braun, R. M., and Primentel,
G.
C.:
Selected Values
q
Physical
and
Thennodynamic Properties of Hydrocarbons and Related Compounds,
Carnegie Preq
Pittsburgh, Pa..
1953.
17.
Mansouri. S. H., and Heywood, J. B.: "Correlation for the Viscosity and Prandtl Number
Hydrocarbon-Air Combustion Products,"
Combust. Sci. Technology,
vol.
23,
pp.
251-256,1980.
18.
Osjara, C., and Borman,
G.
L.:
"A Computer Program for Calculating Properties of Equilibrium
Combustion Products with Some Applications to LC. Engines," SAE paper
750468,1975.
19.
Krieger, R. B., and Borman, G. L.: "The Computation of Apparent Heat Release for Internal
Combustion Engines," in
Proc. Diesel Gas Power,
ASME paper
66-WA/DGP-4,1966.
20.
Martin, M.
K.,
and Heywood. J. B.: "Approximate Relationships for the Thennodynamic Proper.
ties of Hydrocarbon-Air Combustion Products,"
Combust. Sci. Technology,
vol.
15,
pp.
1-la
1977.
21.
Chapman, S., and Cowling, T.
G.:
The Mathematical Theory of Non-Uniform Gases,
Cambrid@
University Press, Cambridge,
1955.
22.
Hirschfelder,
J.
O.,
Curtiss, C. F., and Bud, R. B.:
Molecular Theory of Gases and Liquids.
John
Wdey.
1954.
23.
Patterson,
D.
J., and Henein, N. A.:
Etnissionsfrom Combustion Engines and Their Control,
Ann
Arbor Science Publishers,
1972
24.
D'Alleva,
B.
A.,
and Lovell,
W.
G.:
"Relation of Exhaust Gas Composition to Air-Fuel Ratio."
SAE
J.,
vol.
38,
no.
3,
pp.
90-96,
March
1936.
25.
Stivender.
D.
L.: "Development of a Fuel-Based Mass Emission Measurement Procedure," SAE
paper
710604.1971.
26.
Hamngton, J. A., and Shishu,
R
C.:
"A SingleCylinder Engine Study of the Effects of Fuel Type,
Fuel Stoichiometry and Hydrogen-to-Carbon Ratio on CO, NO, and HC Exhaust Emissions,-
SAE paper
730476,1973.
27.
Spindt, R. S., "Air-Fuel Ratios from Exhaust Gas Analysis," SAE paper
650507,
SAE Trans.,
vol
74. 1965.
28.
Fleming,
R.
D.,
and Eccleston,
D.
B.: "The Effect of Fuel Composition, Equivalence Ratio, and
Mixture Temperature on Exhaust Emissions," SAE paper
710012,1971.
29.
Eltinge,
L.:
"Fuel-Air Ratio and Distribution from Exhaust Gas Composition," SAE paper
6801 14,
SAE Trans.,
vol.
77,1968.
30.
Leonard,
L.
S.: "Fuel Distribution by Exhaust Gas Analysis," SAE paper 379A,
1961.
31.
Bishop, R. P.: "Combustion Efficiency in Internal Combustion Engines," B.S. Thesis, Department
of Mechanical Engineering, MIT, February
1985.
32.
The Engine Test Code Subcommittee of the General Technical Committee,
General Motors Auto-
motive Engine Test Code for Four Cycle Spark Ignition Engines,
6th ed,
1975.
CHAPTER
IDEAL
MODELS
OF
ENGINE
CYCLES
5.1
INTRODUCTION
The operating cycle of an internal combustion engine can be broken down into a
sequence of separate processes: intake, compression, combustion, expansion, and
exhaust. With models for each of these processes, a simulation of a complete
engine cycle can
be
built up which can
be
analysed to provide information on
engine performance. Models of individual engine processes at various levels of
approximation have been developed. In this chapter we consider the simplest set
of
models which provide useful insights into the performance and efficiency of
engines. The cycles analysed are commonly called the constant-volume, constant-
pressure, and limited-pressure cycles; each title describes the approximation
made for the engine combustion
process.t The description of more accurate
Simulations of engine processes is deferred until Chap.
14.
For each engine cycle, a choice of models for working fluid thermodynamic
Properties must
be
made. These models have been reviewed in Chap.
4.
Ideal
t
These
cycles are also referred to by the titles Otto cycle, Diesel cycle, and dual cycle, respectively,
for historical reasons. The more descriptive titles
used
above are preferred
~CC~UK
they avoid the
ohmade assumption that the SI or Otto engine is best approximated by the constant-volume cycle.
&d
the
CI
or diesel engine is best approximated by the constant-pressure cycle. These assumptions
not necessarily
wmct.
162
INTERNAL COMBUSTION ENGINE FUNDAMENTALS
engine cycle models combined with a simple ideal gas model (specific heats con-
stant throughout the engine cycle-model
1
in Table 4.2) provide analytic results
and are useful for illustrative purposes; we will call these cycles
ideal gas standard
cycles.
Ideal engine cycles combined with more realistic models of working fluid
properties (a frozen mixture of ideal gases for the unburned mixture and an equi.
librium mixture for the burned mixture--model
5
in Table 4.2) are called
fuel-air
cycles
and provide more quantitative information on engine operation.
An internal combustion engine is not a heat engine in the thepodynamic
definition of the term. It is not a closed system. The working fluid does not
execute a thermodynamic cycle. The temperature changes which occur around
minimum and maximum cylinder volumes are not primarily a result of heat-
transfer interactions. An engine can best be analyzed as an open system which
exchanges heat and work with its surrounding environment (the atmosphere).
Reactants (fuel and air) flow into the system; products (exhaust gases) flow out.
(An overall second law analysis of the engine from this point of view has already
been presented in
Sec. 3.6.) Thus, the cycles discussed in this chapter are not
thermodynamic cycles. Rather, each is a consecutive sequence of processes
through which we can follow the state of the working fluid as the engine executes
a complete operating cycle.
5.2
IDEAL MODELS
OF
ENGINE PROCESSES
The sequence of processes which make up a typical SI and CI engine operating
cycle has been described in Sec. 1.3. To illustrate these processes, cylinder pres-
sure
(p)
and volume
(V)
data from a throttled four-stroke cycle
SI
engine are
plotted as a
p-V
diagram in Fig. 5-1. The cycle can be divided into compression,
Cylinder volumelV,,
FIGURE
5-1
Pmsure-volume diagram of firing spark-ignition engine.
r,
=
8.4, 3330 rev/min,
pi
=
0.4
atm,
Pe
atm, imep,
=
2.9 atm.
TABLE
5.1
ldcal
models
of
engine
processes
Rarss
kssumptiom
compression (1-2)
1. Adiabatic and reversible (hence isentropic)
combustion (2-3)
1.
Adiabatic
2. Combustion occurs at
(a) Constant volume
(b)
Constant pressure
(c)
Part
at constant volume and part at constant
pressure (dled limited pressure)
3. Combustion
is
complete (q,
=
1)
~xpansion (3-4) 1. Adiabatic and reversible (hence isentropic)
Exhaust
(4-5-6)
1.
Adiabatic
and
2.
Valveevents occur at top and bottomcenter
intake
(6-7-1)
3. NO change in cylinder volume
as
pressure
differences across open valves drop to mo
4.
Inlet and exhaust pressures constant
5.
Velocity effects negligible
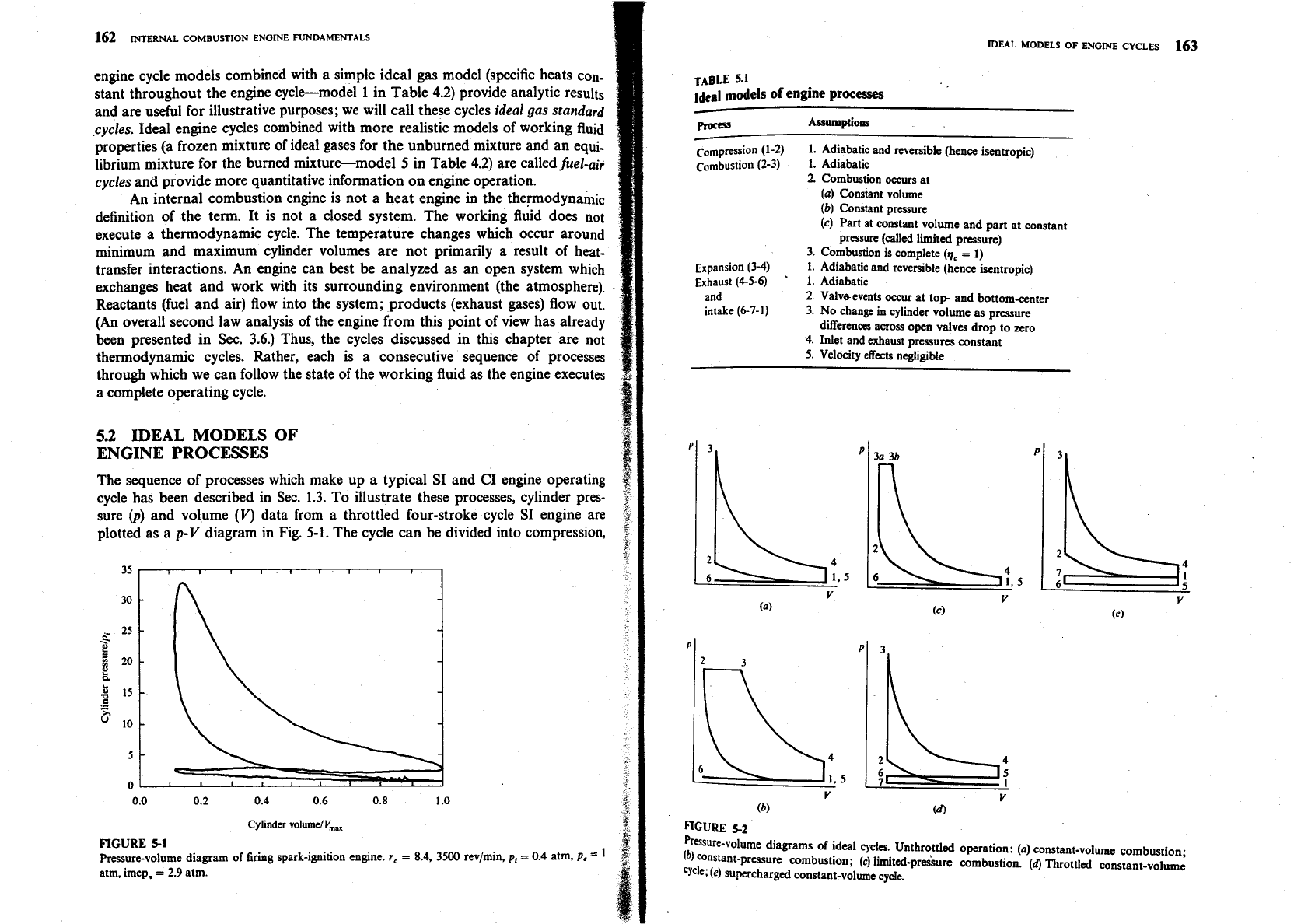
prasure-volume diagrams of ideal cycles. Unthrottled operation: (a) constant-volume combustion;
(b)
constant-pressure combustion;
(c)
limited-pr&urc combustion.
(4
Throttled constant-volume
We;
(e)
supercharged constant-volume cycle.

combustion, expansion, exhaust, and intake processes. Sets of assumptions which
simplify each of these processes to a form convenient for analysis are given in
Table 5.1.
Pressure-volume diagrams for the constant-volume, constant-pressure, and
limited-pressure cycles for unthrottled engine operation are illustrated in Fig.
5-2a to
c.
Throttled engine operation
(pi
<
pJ
and supercharged engine operation
(p,
>
pJ
are shown in Fig. 5-2d and
e.
In
each cycle 1-2 is the compression
process, 2-3 is the combustion process,
3-4
(or 2-4 in the constant-pressure cycle)
is the expansion process, 4-5-6 is the exhaust process, and 6-7-1 is the intake
process.
The most critical assumption in determining how useful these ideal cycles
are as indicators of engine performance is the form assumed for the combustion
process. The real engine combustion process occupies a finite crank angle period
(between about 20 and 70 crank angle degrees), and the spark or fuel-injection
timing may be retarded from its optimum advance to closer to
TC.
The constant-
volume cycle is the limiting case of infinitely fast combustion at TC; the constant-
pressure cycle would correspond to slow and late combustion; the limited-
pressure cycle lies in between.
53
THERMODYNAMIC RELATIONS
FOR ENGINE PROCESSES
The overall engine operating parameters of greatest interest which can be deter-
mined from a thermodynamic analysis of the engine operating cycle are:
The indicated fuel conversion efficiency
v,,~:
(which, since the combustion efficiency is unity, is equal to the indicated thermal
conversion efficiency
v,,~;
see Sec. 3.6.2)
The indicated mean effective pressure (imep):
W,,,
,
the indicated work per cycle, is the sum of the compression stroke work and
the expansion stroke work:
Using the notation of Fig. 5-2 to define the endpoints of each engine process, the
following relationships are obtained by applying the fist and second laws of
thermodynamics to the cylinder contents:
compression stroke:
"1
-
=
rc
u2
Since the process is adiabatic
and
reversible
S2
=
S1
The compression work is
Combustion process:
For the constant-volume cycle,
"3
=
u2
For the constant-pressure cycle,
P3
=
P2
For the limited-pressure cycle,
and
Expansion stroke:
For the constant-volume cycle,
and the expansion work is
WE
=
U3
-
U4
=
m(u3
-
14,)
For the constant-pressure cycle,
"4
-
rc s4
=
s3
P3
=
P2
-
-
"2
and the expansion work is

166
INTERNAL COMBUSTION ENGINE FUNDAMENTALS
For the limited-pressure cycle,
b
vdu3a
=
P3b
=
P3a S4
=
S3b
and the expansion work is
=
u3b
-
u4
+
~3(~3b
-
v33
=
mC(u3b
-
~4)
+
~3(~3b
-
v3lr)I
=
mC(h3b
-
h4)
+
P4 v4
-
P3 03al
The indicated fuel conversion efficiency is found by substitution into
Eqs.
(5.3)
and
(5.1):
P4
FIGURE
5-3
Enthalpyzntropy diagram of gas state during
s
exhaust process.
See
text for explanation.
For the constant-volume cycle:
mC(u3
-
u3
-
(~2
-
u1)1
gas to leave have the same stat-5. There is, therefore, a gradient in temperature
41.1
=
within the exhausted gas. The temperature of the first element exhausted,
T,,
is
mf
QLHV
slightly less than
T,;
the temperature of the last element exhausted is
T5
.
For the constant-pressure cycle:
A
displacement of gas out of the cylinder follows the blowdown process as
the piston moves from
BC
to TC.
If
heat-transfer and kinetic energy dissipation
mC(:(h3
-
h4)
-
(u2
-
~1)
+
~4 v4
-
~2
v21
ekts are neglected, no change in thermodynamic state of the gas occurs. In this
4f.i
=
m,
QLHV
displacement
process, the mass of gas within the cylinder at the end of the blow-
down process is further decreased by the ratio
&/&.
For the limited-pressure cycle:
The mass of residual gas
m,
in
the cylinder at point
6
in the cycle is
rnC(h3b
-
h3
-
(%
-
~1)
+
P4 04
-
P3 u3aI
obtained by first determining the state of the gas
(T,,
us)
at the end of the blow-
41.i
=
down process following an isentropic expansion from
p4
to
pe
and then by
mf
QLHV
reducing the cylinder volume to the clearance volume
V,.
The residual mass
The state of the mixture at point
1
in the cycle depends on the intake
fraction is thus given by
mixture properties and the residual gas properties at the end of the exhaust
stroke.
m,
vdvs
02
-
-=x,=---
m
(5.17)
When the exhaust valve opens at point 4, the cylinder pressure is above the
rc
us
exhaust manifold pressure and a
blowdown
process occurs. In the ideal exhaust
The average state of the exhausted gas can
be
determined by considering
process model, this blowdown occurs with the piston stationary at BC. During
the
Open system defined by the piston face, cylinder walls, and cylinder head,
this blowdown process, the gas which remains inside the cylinder expands isen-
shown in Fig. 5-4. Applying the first law of thermodynamics for an open system
tropically. The gases escaping from the cylinder undergo an unrestrained expan-
gives
sion or throttling process which is irreversible. It is assumed that the kinetic
energy acquired by each gas element as it is accelerated through the
exhaust
U6
-
U4
=
p&V4
-
V,)
-
He
(5.18~)
valve is dissipated in a turbulent mixing process in the exhaust port into internal
energy and flow work. Since it is also assumed that no heat transfer occurs, the
where
He
is the enthalpy of the mass of gas exhausted from the cylinder. The
s~ecific exhaust enthalpy is, therefore,
enthalpy of each element of gas after it leaves the cylinder remains constant.
These processes are illustrated on an
h-s
diagram
in
Fig. 5-3.
he
gas
-
m4"4-m6u6+~e6
remaining in the cylinder expands isentropically along the line 4-5. The
fint
he
=
(5.18b)
"'4
-
m6
element of gas which leaves the cylinder at point 4 enters the exhaust manifold
at
state
a
on the pressure
=
pe
line. An element that leaves the cylinder at an inter-
which, with
p
=
pe,
defines the average exhausted-gas state.
mediate state
b
on the expansion line 4-5 would enter the exhaust manifold
at
The
mixture temperature at the end of the intake stroke and at the start of
state
c.
At the end of the blowdown process the gas in the cylinder and the last
Ihe
stroke (point
1
in Fig. 5-2) can now be determind, again using

Exhaust
-
'I
Definition of system boundary for thermodynamic
analysis of ideal cycle processes.
the open system in Fig.
5-4.
Application of the first law between points
6
and 1
gives
U1
-
U6
=
-pi(Vl
-
V6)
+
(ml
-
m6)hi
(5.19~)
where
hi
is the specific enthalpy of the inlet mixture and
p,
=
pi.
Note that when
pi
<
pe,
part of the residual gas in the cylinder at the end of
the exhaust stroke will flow into the intake system when the intake valve opens.
This flow will cease when the cylinder pressure equals
pi.
However, provided
no
heat transfer occurs, this backflow will not affect Eqs.
(5.19)
above, since the flow
of residual through the intake valve is a constant enthalpy process.
In many engines, the closing of the exhaust valve and the opening of the
intake valve overlap. Flow of exhausted gases from the exhaust system through
the cylinder into the intake system can then occur. Equations
(5.18)
and
(5.19)
would have to
be
modified to account for valve overlap.
In the four-stroke engine cycle, work is done on the piston during the
intake and the exhaust processes. The work done by the cylinder gases on the
piston during exhaust is
The work done by the cylinder gases on the piston during intake is
W
=
PXVI
-
V2)
(5.21)
The net work to the piston over the exhaust and intake strokes, the
pumping
work,
is
Wp
=
(Pi
-
P~XVI
-
V2)
(5.22)
which, for the cylinder gas system, is negative for
pi
<
pe
and positive for
pi
>
PI-
fie ~umping mean effective pressure (pmep) is usually defined as a positive
Thus:
For
Pi
<
Pe:
For
pi
>
pe:
The net and gross indicated mean effective pressures are related by
The net indicated fuel conversion efficie;cy is related to the gross indicated fuel
conversion efficiency by
5.4
CYCLE ANALYSIS WITH
IDEAL
GAS
WORKING
FLUID
WITH
c,
AND
c,
CONSTANT
If
the working fluid in these ideal cycles is assumed to
be
an ideal gas, with
cu
and
c,
constant throughout the engine operating cycle, the equations developed in the
previous section which describe engine performance and efficiency can be further
simplified. We will use the notation of Fig.
5-2.
5.4.1
Constant-Volume Cycle
The compression work (Eq.
5.6)
becomes
Wc
=
mc,,(Tl
-
T2)
The expansion work (Eq.
5.9)
becomes
WE
=
mc,,(T3
-
T,)
The denominator in Eq.
(5.14),
m,
QLHv, can be related to the temperature
rise during combustion. For the working fluid model under consideration, the
U(T)
lines for the reactants and products on a
U-T
diagram such as Fig.
3-5
are
parallel and have equal slopes, of magnitude
c,.
Hence, for a constant-volume
adiabatic combustion process
t
Note that
if
insutficient air is available for complete combustion of the fuel,
Eq.
(5.28)
must
be
modified. The right-hand side of the equation should then
be
E
m,QLHv.
where
E
is the combustion
dtiency given by
Eq.
(3.27).

Note that the heating values at constant volume and constant pressure are the
same for this working fluid. For convenience we will define
Q* is the specific internal energy (and enthalpy) decrease, during isothermal com.
bustion, per unit mass of working fluid.
The relation for indicated fuel conversion efficiency (Eq. 5.14) becomes
Since 1-2 and
3-4
are isentropic processes between the same volumes,
Vl
and
V2,
where
y
=
c Jc, . Hence:
and Eq. (5.30) can be rearranged as
Values of q,,, for different values of
y
are shown in Fig. 5-5. The indicated
fuel conversion efficiency increases with increasing compression ratio and
decreases as
y
decreases.
FIGURE
5-5
Ideal gas constant-volume cycle
fuel
con-
version dficiency
as
a function of
corn-
pression ratio;
y
=
E#,.
The indicated mean effective pressure, using Eqs. (5.2) and (5.31), becomes
The dimensionless numbers
r,,
y,
and Q*/(c,T,) are sufficient to describe
[he characteristics of the constant-volume ideal gas standard cycle, relative to its
initial conditions pl, TI.
1t
is useful to compare the imep-a measure of the effectiveness with which
[he displaced volume of the engine is used to produce work-and the maximum
pressure in the cycle, p3. The ratio p31pl can be determined from the ideal gas
applied at points
2
and 3, and the relation
obtained from Eq. (5.28). Equations (5.32) and (5.33) then give
A
high value of imeplp, is desirable. Engine weight will increase with increasing
p,
to withstand the increasing stresses in components.
The indicated fuel conversion efficiency and the ratios imeplp, and imep/p3
for this ideal cycle model do not depend on whether the cycle is throttled or
supercharged. However, the relationships between the working fluid properties at
points 1 and
6
do depend on the degree of throttling or supercharging. For throt-
tled engine operation, the residual gas mass fraction
x,
can be determined as
follows. From Eq. (5.17), since state 5 corresponds to an isentropic expansion
from
state
4
to p
=
p,,
x,
is given by
it
follows
that
The residual mass fraction increases as pi decreases below pe, decreases as
r,
increases, and decreases as Q*/(c,
T,)
increases.
Through a similar analysis, the temperature of the residual gas T, can be
determined:
