Henini M. Handbook of Self Assembled Semiconductor Nanostructures for Novel devices in Photonics and Electronics
Подождите немного. Документ загружается.


PbSe Core, PbSe/PbS and PbSe/PbSe
x
S
1
x
Core–Shell Nanocrystal
Quantum Dots: Properties and Applications
E. Lifshitz ,
1
A. Kigel,
1
M. Brumer,
1
L. Etgar
,
V. Kloper , M. Bashouti ,
1
A. Sashchiuk,
1
R. Tennenbaum,
2
M. Sirota
,
3
E. Galun
,
3
Z. Burshtein,
4
A.Q. Le Quang,
5
I. Ledoux-Rak,
5
and J. Zyss
5
1
Shulich Faculty of Chemistry, Solid State Institute and the Russell Berrie
Nanotechnology Institute, Technion, Haifa 32000, Israel ;
2
Department of Chemical Engineering, Russell Berrie Nanotechnology Institute,
Technion, Haifa 32000, Israel;
3
ElOp Electro Optics Industries Ltd, PO Box 1165 Rehovot 76111, Israel;
4
Department of Materials Engineering, Ben-Gurion University of the Negev,
PO Box 653, Beer-Sheva 84105, Israel;
5
Laboratoire de Photonique Quantique et Moléculaire, Institut d Alembert, Ecole Normale
Supérieure de Cachan, 61 avenue du Président Wilson 94235 Cachan, France
25.1 Introduction
Colloidal PbSe nanocrystal quantum dots (NQDs) have currently received special interest due
to their unique electronic and optical properties [1] . The bulk PbSe semiconductor has a rock-
salt crystal structure, and a narrow direct band gap (0.28 eV at 300 K) with both valence and
conduction band maxima being four-fold degenerate at the L-point of the Brillouin zone. Also,
the PbSe semiconductor possesses a high dielectric constant (ε
18.0) and a relatively large
effective Bohr radius ( a
B(PbSe)
46 nm). Previous k ⴢ p calculations of the electronic structure of
the PbSe NQDs suggested an ordinary effective mass, such as S, P, and D states, with similar low
masses for the electron and hole, and consequently a mirror-like density of states between the
valence and conduction bands [2–4] . Recent use of the tight binding model [5] and the atomis-
tic pseudopotential [6] method have taken into consideration the strong anisotropy of the bulk
L-valleys with transverse and longitudinal effective masses [4] , and valley-to-valley coupling:
both issues split the bulk degenerate L-states, leading to a densely spaced hole manifold.
Interband optical studies of colloidal PbSe NQDs exhibit well-defi ned band edge excitonic tran-
sitions tuned between 0.8 and 4 μ m [7–10] . Photoluminescence (PL) lifetime measurements and
transient absorption measurements revealed an intraband relaxation process on the order of
ps, and an interband recombination emission on the order of hundreds of ns [8–10] . Recently,
amplifi ed spontaneous emission from PbSe NQDs was demonstrated [9] with gain parameters
similar to those observed in CdSe NQDs [11] . Furthermore, the impact ionization process in PbSe
NQDs, obtained upon photoexcitation with h ν 3 E
g
and its competition with Auger recombi-
nation have been discussed [3, 10] . The dependence of the transient optical absorption on the
power of a pumping laser was measured by Klimov et al. [9] , suggesting the creation of multi-
ple excitons (up to eight electron–hole pairs), occupying the four valleys at the L-point of the
CHAPTER 25
CH025-I046325.indd 749CH025-I046325.indd 749 6/25/2008 11:16:18 AM6/25/2008 11:16:18 AM
750 Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics
Brillouin zone of PbSe NQDs, with an anticipated benefi t in gain devices. Thus, the aforemen-
tioned investigations indicate a widespread interest in the unique physical properties of PbSe
NQDs, and the feasibility of using the PbSe NQDs in telecommunications [12] , near-infrared (IR)
lasers [13] , and as biological markers [14, 15] .
Various colloidal syntheses have been developed in the last couple of years, producing PbSe
NQDs with size monodispersity ( 5% size distribution), uniform shape, and high crystallinity.
Murray et al. [16] and Colvin et al. [17] synthesized spherical core PbSe NQDs, soluble in organic
or water solutions, with narrow size distributions and band gap tuning at the near-IR spec-
tral regime. Lifshitz et al. [18] reported a colloidal procedure for the preparation of spherical
PbSe/PbS core–shell NQDs, with an average size ranging between 2.5 and 7.0 nm, using tri-
butylphonsphine/tri-octylphosphine (TBP/TOP) surfactants. Lifshitz et al. [19, 20] also reported
recently the formation of unique PbSe/PbSe
x
S
1
x
core-alloyed–shell NQDs with tunable composi-
tion of the alloyed shell (using oleic acid (OA) and TOP surfactants), showing exceptionally high-
luminescence quantum effi ciency (QE). Synthesis of core–inorganic shell NQDs is usually carried
out to increase the air and luminescence stability; various synthesis methods have recently
been reported for PbSe/PbS core–shell NQDs [21, 22] . Lifshitz et al. [23] used alkyldiamine or
ethylene glycol as a coordinating molecular template, which led to the formation of PbSe wires
(20 nm 1 μ m), rods (20 nm 100 nm), ribbons (60 nm 0 . 5 μ m), stars (500 nm) and cubes
(100 nm). Furthermore, Lifshitz et al. [24] reported the formation of one-dimensional assemblies
composed of spherical PbSe NQDs, showing high conductivity properties. The conductivity prop-
erties of PbSe NQDs ’ arrays were examined recently at low temperatures by Wehrenberg et al.
[25] and at high temperatures by Drndic et al. [26] .
This chapter discusses a few alternative synthetic routes for the synthesis of chemical stable
spherical and colloidal PbSe NQDs, synthesized as PbSe cores, PbSe/PbS core–shell structures,
and PbSe/PbSe
x
S
1
x
core-alloyed–shell structures (section 25.2). A thorough investigation
of the structural and optical properties of the indicated NQDs is given below (section 25.3 and
25.4), suggesting the formation of NQDs with QE up to 65%, relatively narrow emission bands, a
peculiar Stokes-shift behaviour and an excited-state lifetime ranging between 70 ns and 900 ns,
depending on the pumping power intensity, composition and size of the NQDs. The discussed
NQDs were used as passive Q-switches in an eye-safe laser of Er:glass (section 25.5), acting as
“ fast ” saturable absorbers with a relatively large ground-state cross-section of absorption. In
addition, the gain properties of the discussed NQDs were examined, showing an amplifi ed spon-
taneous emission (ASE) under conditions that are suitable for technological devices, such as opti-
cal pumping by a continuous diode laser under room temperature conditions (section 25.6). The
attachment of the PbSe NQDs to metallic particles (Fe
2
O
3
) is discussed in section 25.7, propos-
ing the use of the hybrid structures as biological transport and tagging agents. The conductiv-
ity properties of PbSe NQDs self-assembled solids, annealed at 200°C, were examined, showing
an ohmic behaviour at the measured voltages (up to 30 volts), which is governed by a variable
range-hopping transport mechanism (section 25.8).
25.2 Synthesis, chemical stability, and structural characterization of PbSe NQDs, PbSe/PbS
core–shell NQDs and PbSe/PbS
x
eS
1 x
core-alloyed–shell NQDs
25.2.1 Synthesis of PbSe NQDs cores, covered with organic surfactants (alternative I)
The synthesis of core PbSe NQDs followed a modifi ed procedure to that given by Murray et al.
[16] , following the procedure given in [19] and including the preceding stages: (1) 0.71 gr of
lead(II) acetate trihydrate [Pb-ac] (Pb[CH
3
COO]
2
· 3H
2
O, GR, Merck) were dissolved in a solution
composed of 2 mL diphenyl ether [PhEt] (C
6
H
5
OC
6
H
5
, 99%, Aldrich), 1.5 mL OA (CH
3
(CH
2
)
7
CHCH(CH
2
)
7
COOH, 99.8%, Aldrich) and 8 mL TOP ((C
8
H
17
)
3
P, Tech, Aldrich), under stand-
ard inert conditions in the glove box, and were inserted into a three-neck fl ask (fl ask I); (2)
10 mL of PhEt were inserted into a three-neck fl ask (fl ask II) under the inert conditions of a
glove box; (3) both fl asks were taken out of the glove box, placed on a Schlenk line and heated
under a vacuum to 100 120°C for an hour; (4) fl ask I was cooled to 45°C, while fl ask II was
CH025-I046325.indd 750CH025-I046325.indd 750 6/25/2008 11:16:19 AM6/25/2008 11:16:19 AM
PbSe Core, PbSe/PbS and PbSe/PbSe
x
S
1 x
Core–Shell Nanocrystal Quantum Dots 751
heated to 180 210°C, both under a fl edging of an argon gas; (5) 0.155 gr of selenium powder
(Se, 99.995%, Aldrich) was dissolved in 2.0 mL TOP, forming a TOP:Se solution, under standard
inert conditions of a glove box. Then, 1.7 mL of this solution was injected into fl ask I on the
Schlenk line; (6) the content of fl ask I, containing the reaction precursors, was injected rapidly
into the PhEt solution in fl ask II, reducing its temperature to 100 130°C, leading to the forma-
tion of PbSe NQDs within the fi rst 15 min of the reaction. The described procedure produced
nearly mono-dispersed NQDs with 8% size distribution, with average size between 3 and 9 nm,
controlled by the temperature and by the time duration of the reaction.
25.2.2 Synthesis of PbSe NQDs cores, covered with organic surfactants (alternative II)
In alternative II, the PbSe core NQDs were synthesized according to a procedure given by Colvin
et al. [17] . A mixture of 0.892 g of PbO (4.00 mmol), 2.825 g of OA (10.00 mmol), and techno-
logical grade 1-octadecene (ODE) (the total weight was 16 gr) was initially heated to 150°C and
after the mixer was turned colourless, it was further heated to 180°C. Then, 6.4 gr of Se-TOP
solution (containing 0.64 gr of selenium, 8.00 mmol) was quickly injected into the PbO hot solu-
tion. The temperature of the reaction mixture was allowed to cool to 150°C for the growth of the
PbSe NQDs. All steps in the reactions were carried out under argon.
25.2.3 Synthesis of PbSe/PbS core–shell NQDs by a two-injection process
The preparation of PbSe/PbS core–shell NQDs by a two-injection process begins with formation
of core PbSe NQDs and their isolation from the initial reaction solution, according to the pro-
cedure in section 25.2.1. Those core NQDs were re-dissolved in chloroform solution, forming a
solution of 50 mg/mL weight concentration. A quantity of 1.4 mL of TOP was then added to the
NQDs solution, while the chloroform molecules were removed by distillation under vacuum and
heating at 60°C. In parallel, 0.2 gr of a Pb precursor, Pb-ac, was dissolved in a mixture of 2 mL
PhEt, 1.5 mL of OA, and 8 mL of TOP, heated to 120°C for an hour, and then cooled to 45°C.
Also, 0.03–0.10 gr of sulphur (S, 99.99 %, Aldrich) was dissolved in 0.3 mL of TOP and was
premixed with a PbSe core NQDs in a TOP solution. This mixture was injected into the Pb-ac
solution. All reagents were then injected into a PhEt mother solution and kept on a Schlenk line
at 180°C, causing a reduction in temperature of the mother solution to 120°C. The indicated
chemical portions caused the precipitation of 1–3 monolayers (MLs) of PbS shell over the PbSe
core surface within the fi rst 15 min of the reaction.
25.2.4 Synthesis of PbSe/PbSe
x
S
1
x
core-alloyed–shell NQDs by a single-injection process
The preparation of PbSe/PbSe
x
S
1
x
core-alloyed–shell structures was nearly identical to that of
the core PbSe NQDs, described in section 25.2.1 using a single injection of the precursors into
a single round fl ask. However, step (5) was altered by the use of an alternative chalcogen pre-
cursor stock solution. A stock solution of Se and S was prepared by mixing 0.15 gr Se dissolved
in 1.4 mL TOP, with 0.03–0.10 gr S dissolved in 0.3 mL TOP. The amount of S in the new stock
solution corresponded to a stoichiometric amount of 1–2 ML of the PbS compound. Thus, the
mole ratio of the precursors Pb:Se:S ranged from 1:1:0.5 to 1:1:1.3.
Aliquots were drawn periodically from the mother solutions described in sections 25.2.1–
25.2.4. A quenching process to room temperature terminated the NQDs ’ growth. They were iso-
lated from the aliquots solution by the addition of methanol, and by centrifugation. The isolated
NQDs were further purifi ed by dissolving them in chloroform, followed by fi ltering several times
through a 0.02 micron membrane. The purifi ed NQDs were examined by structural analyses,
absorption and PL spectroscopy.
25.2.5 PbSe core and core–shell NQDs, capped with water-soluble ligands
The organic capping of the PbSe core and core–shell NQDs could be replaced with strong polar
groups such as 2-aminoethanethiol ([HS(CH
2
)
2
NH
3
)], AET) ligands using a procedure described
CH025-I046325.indd 751CH025-I046325.indd 751 6/25/2008 11:16:19 AM6/25/2008 11:16:19 AM
752 Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics
elsewhere [27] , converting the NQDs into water-soluble species. To create water-soluble PbSe
NQDs with a positively charged capping, 100 μ L of an organically capped PbSe NQD solution was
dissolved in 5 mL of chloroform. Subsequently, 100 μ L of a 0.5 M methanol solution of AET was
added. The relatively stronger affi nity of the thiol group originating from AET ligands to the PbSe
surface, with respect to that of the OA carboxyl group, led to a ligand exchange. Thus, the exte-
rior surfaces of the PbSe NQDs exchanged aliphatic terminating groups (the CH
3
end-groups of
the OA) with the amine groups of the AET ligands. This immediately caused fl occulation of the
PbSe NQDs in chloroform. Subsequently, 5 mL of water was added to the suspension, resulting in
a separation of the mixer into two phases (water above chloroform). Upon further shaking, the
fl occulated NQDs dissolved into the water phase and formed a clear suspension.
25.2.6 Storage conditions and encapsulation of the NQDs in a polymer fi lm
The colloidal NQDs were embedded in a polymer fi lm or dissolved in a glassy solution
(2,2,4,4,6,8,8,-heptamethyl-nonane) for the optical measurements. The polymer was pre-
pared by mixing PbSe NQDs in chloroform solution with poly-methyl-methacrylate (PMMA)
([–CH
2
C(CH
3
)(CO
2
CH
3
)–]
n
, analytical grade, Aldrich) polymer solution. The resultant mixture
was spread on a quartz substrate and dried over 24 h to a uniform fi lm.
The organically capped PbSe NQDs were stored either in a hexane solution or as a dry powder
in air or in nitrogen ambient. The stability of these NQDs was examined over a period of time
by recording the absorption spectra (see section 25.4) and following the consistency of the low
exciton’s energy. Plots of this exciton energy versus time suggest that the exciton energy in the
core samples is blue shifted by ⬃400 meV over a period of days for the NQDs kept in a chloroform
solution. Such a blue shift, however, occurs over months for the samples kept as dry powders.
On the other hand, the energy shift is smaller for the PbSe/PbS core–shell samples, and even
nearly disappeared for the NQDs coated with three PbS shell MLs. It is presumed that the exci-
ton energy blue shift is due to oxidation of the surface, and a decrease of the effective size of the
core. Obviously, the penetration of oxygen through the PbS shell is reduced as the shell becomes
thicker. Furthermore, storage of the NQDs in a nitrogen environment nearly eliminates any spec-
tral drift over a period of months, even extending to two or three years.
25.2.7 Synthesis of organically capped and water-soluble γ -Fe
2
O
3
magnetic nanoparticles
The synthesis of γ -Fe
2
O
3
nanoparticles (NPs) which were used for the preparation of PbSe NQD’s
γ -Fe
2
O
3
NPs ’ conjugated structure, covered with organic surfactant followed a modifi ed proce-
dure to that documented by Held et al. [28] . A quantity of 0.2 mL of Fe(CO)
5
(1.52 mmol) was
added to a mixture containing 10 mL of octyl ether and 1.28 g of OA (4.56 mmol) at 100°C. The
resulting mixture was heated to 300°C and kept at that temperature for 1 h. During this time, the
initial orange colour of the solution gradually changed into a black colour. The resulting black
solution was cooled to room temperature and was centrifuged. The precipitate was removed, the
remaining supernatant solution was then collected, and ethanol was added to produce reversible
fl occulation of the NPs. The solution was centrifuged again, and the precipitate was saved and
subsequently suspended in hexane to produce a suspension of 6 nm iron NPs. Exposing this sus-
pension to air for several days resulted in the complete oxidation of the iron NPs into dispersed
5 nm γ -Fe
2
O
3
NPs.
The mentioned γ -Fe
2
O
3
NPs were transferred into a water-soluble solution, using the proce-
dure documented by Rotello et al. [29] . A quantity of 10 mg of iron oxide NPs were dissolved in
2 mL of hexane and 100 mg of polyhedral silsesquioxane (PSS) hydrate octakis were dissolved
in 2 mL of water. These solutions were mixed; the solute weight ratio was 10:1. The structure
of PSS hydrate octakis (C
32
H
96
N
8
O
20
Si
8
· xH
2
O) molecules is an octahedral featuring eight siloxy
groups, therefore the resulting water-soluble γ -Fe
2
O
3
NPs were capped by a negative charge. This
bilayer system stayed under an inert environment for 2–3 min, and was then stirred rapidly for
24 h during which the NPs were transferred to the aqueous phase. The solution phases were
carefully separated and the water-soluble iron oxide NPs were run through a 0.22 μ m fi lter.
CH025-I046325.indd 752CH025-I046325.indd 752 6/25/2008 11:16:19 AM6/25/2008 11:16:19 AM
PbSe Core, PbSe/PbS and PbSe/PbSe
x
S
1 x
Core–Shell Nanocrystal Quantum Dots 753
25.3 Structural characterization of PbSe core, PbSe/PbS core–shell and PbSe/PbS
x
eS
1
x
core-alloyed–Shell NQDs
The morphology and crystallography of the colloidal NQDs were examined by transmission
electron microscopy (TEM), high-resolution TEM (HR-TEM) and selected-area electron diffrac-
tion (SAED). High spatial frequency noise of the SAED image was fi ltered out using the built-in
software of the measuring system, based on the Fourier transform of the original image. The
TEM specimens were prepared by injecting small liquid droplets of the solution on a copper grid
(300 mesh) coated with amorphous carbon fi lm and then dried at room temperature.
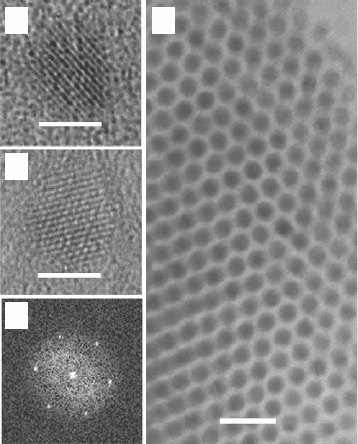
Figure 25.1a shows an HR-TEM image of PbSe/PbS core–shell NQD with an overall diameter of
6.0 nm and a core diameter of 4.8 nm. The HR-TEM image of PbSe/PbSe
x
S
1
x
core-alloyed–shell
NQD, with an overall diameter of 6.0 nm, is presented in Fig. 25.1b . These images reveal the
formation of spherical NQDs with well-resolved crystal planes, without distinct boundaries at
the core–shell interface due to the close matching (⬃3%) of the PbSe and PbS crystallographic
parameters. The increase in the core–shell NQDs ’ size by 1.2 nm as compared to the preliminary
core NQDs is consistent with a PbS or PbSe
x
S
1
x
shell thickness of 1 ML. Figure 25.1c provides
a fast Fourier transform image, corresponding to the measured SAED of the sample shown in
Fig. 25.1a , revealing a cubic single crystal, with lattice spacing of 6.12 Å indicating the exist-
ence of rock salt structure of space group Fm3
–
m. Figure 25.1d presents a TEM image of PbSe/
PbSe
x
S
1
x
NQDs that are 6.7 nm in diameter, capped with organic ligands. The ensemble of
NQDs in the image exhibited a diameter distribution of 5%, self-organized into a hexagonal array.
(a) (d)
(b)
(c)
Figure 25.1 (a) HR-TEM image of a PbSe/PbS (4.8 nm/1.2 nm) core–shell NQD (bar scale 5 n m ) .
(b) HR-TEM image of a PbSe/PbSe
x
S
1
x
core-alloyed–shell NQD ( x 0.5, bar scale 5 nm). (c) Fast Fourier trans-
form image of a measured SAED of the sample in A. (d) TEM image of 6.7 nm PbSe/PbSe
x
S
1
x
core-alloyed–shell
NQDs self-organized on a TEM grid (bar scale 20 nm).
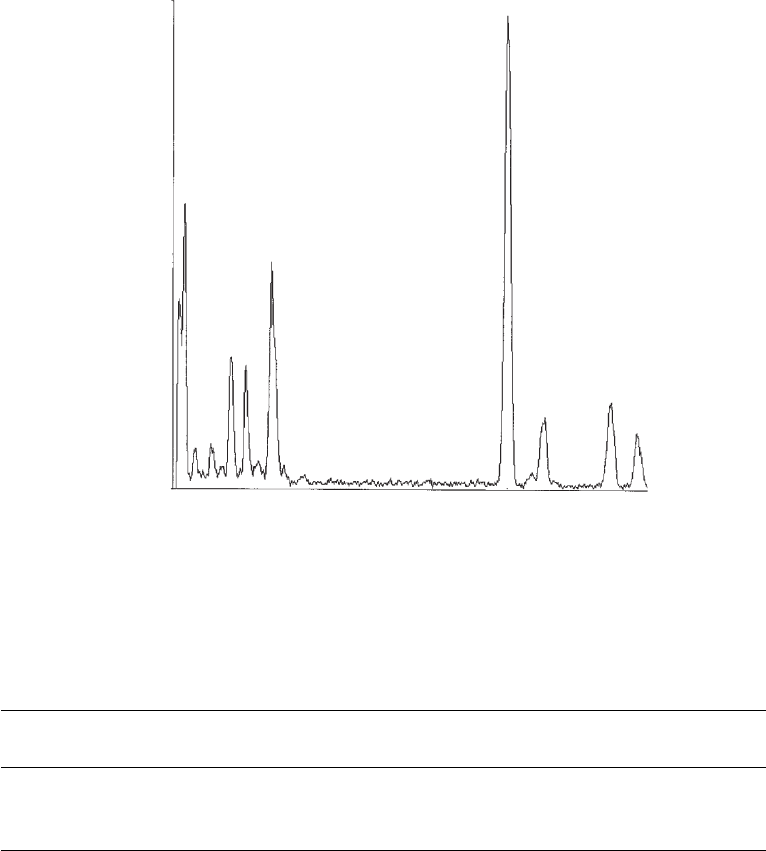
A representative energy-dispersive analysis of an X-ray (EDAX) of the PbSe/PbSe
x
S
1
x
NQDs,
prepared by the procedure given in section 25.2.4, is shown in Fig. 25.2 . The Pb, Se and S per-
centages of aliquots, drawn from the reaction solution after 5 min and after 15 min are compared
in Table 25.1 . Comparison of the atomic percentages of the elements given in the table and the
equivalent molecular formulas of the PbSe/PbSe
x
S
1
x
NQDs suggests that a single injection of
CH025-I046325.indd 753CH025-I046325.indd 753 6/25/2008 11:16:19 AM6/25/2008 11:16:19 AM
754 Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics
Pb, Se and S constituents starts with embryonic nucleation of PbSe cores, followed by the forma-
tion of PbSe
x
S
1
x
alloyed shells with variable sulphur contents.
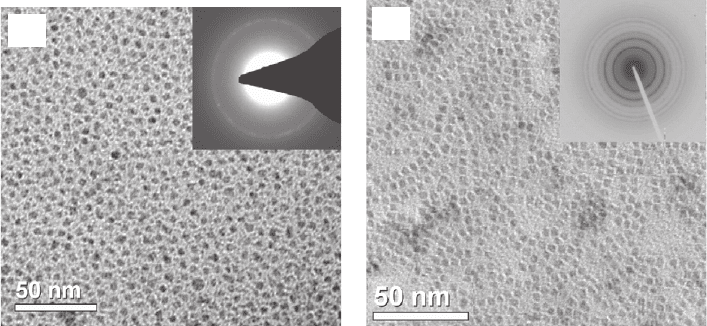
Figure 25.3 compares the TEM images of PbSe core NQDs, with an NQD average diameter of
6.0 nm, capped either with OA (a), or with AET (b). Comparison between the two images suggests
that the process has not altered the size, shape and disparity of the NQDs before and after the
ligand exchange. The water-soluble AET-capped NQDs exhibited a high chemical stability under
a nitrogen atmosphere for an extended period of time (over months), without any indication of
aggregation or photo-degradation. The electron diffraction pattern of the PbSe core NQD sample
in an aqueous medium (insets in Fig. 25.3a and b ) confi rms that the NQDs have a perfect cubic
close-packed (ccp/fcc) crystal structure with excellent agreement with the cubic (rock salt) struc-
ture of bulk PbSe and of the NQDs capped with organic ligands. The FTIR spectra (given in [27] )
of the AET-capped NQDs in the aqueous solution exhibited two distinct bands, centred at 3469.3
and 3346.7 cm
1
, corresponding, respectively, to the asymmetric and symmetric stretching
300
Cu
Pb
Pb
s
s
Cu
Cu
Se
Se
Se
Si
c
o
u
n
t
s
C
O
P
11.520
KeV
0.000
0
Figure 25.2 EDAX spectra of PbSe/PbSe
x
S
1 x
core-alloyed–shell NQDs, prepared with initial Pb:Se:S 1: 1:0.5
and extricated from the solution after 15 min.
Table 25.1 Atomic percentages of Pb, Se and S constituents in PbSe/PbSe
x
S
1
x
NQDs,
as derived from the EDAX measurements
Aliquots
(elapsing time)
Initial Pb:Se:S
molar ratio
Pb (%) Se (%) S (%) NC molecular
formula
5 minutes 1:1:0.5 51 49 – PbSe
15 minutes 1:1:0.5 52.1 41.8 6.1 PbSe/PbSe
0.7
S
0.3
15 minutes 1:1:1.3 46.7 22.6 30.7 PbSe/PbSe
0.1
S
0.9
CH025-I046325.indd 754CH025-I046325.indd 754 6/25/2008 11:16:20 AM6/25/2008 11:16:20 AM
PbSe Core, PbSe/PbS and PbSe/PbSe
x
S
1 x
Core–Shell Nanocrystal Quantum Dots 755
vibrations of terminal N–H bonds, suggesting the existence of free amine groups at the exterior
side of the NQDs.
25.4 Optical properties of PbSe NQDs, PbSe/PbS core–shell NQDs and PbSe/PbS
x
eS
1
x
core-alloyed–shell NQDs
Absorbance spectra were recorded using a UV-vis-near-IR spectrometer. PL spectra were obtained
by exciting the samples with energy either above the band gap, using a Ti:sapphire laser (operat-
ing at 690–840 nm), or tuned with the band gap, using a near-IR laser diode (operating at 1463–
1577 nm). The resonance excitation allowed the recording of FLN spectra. A monochromator
and liquid nitrogen-cooled InSb detector detected the emission processes. All measurements were
carried out at room temperature.
The PL QE was measured using an integrating sphere technique, described by de Mello et al.
[30] . A solution of NQDs was placed inside an integrating sphere and excited by a monochro-
matic light. Luminescence spectra were detected by a fi bre-coupled spectrometer equipped with
a liquid nitrogen-cooled Ge photo detector. The entire system response was normalized against
a calibrated detector, and care was taken to ensure that the sample absorption was more than
20%. The lifetime measurements were performed at room temperature using a pulsed (10Hz)
1.064 μ m ND-YAG laser, with 4.3 ns pulsewidth. For the detection we used an Acton monochro-
mator conjugated with a Hammamtsu InGaAs PMT. We recorded the spectra using a scope, syn-
chronized with the laser system and averaged over a given number laser pulses.
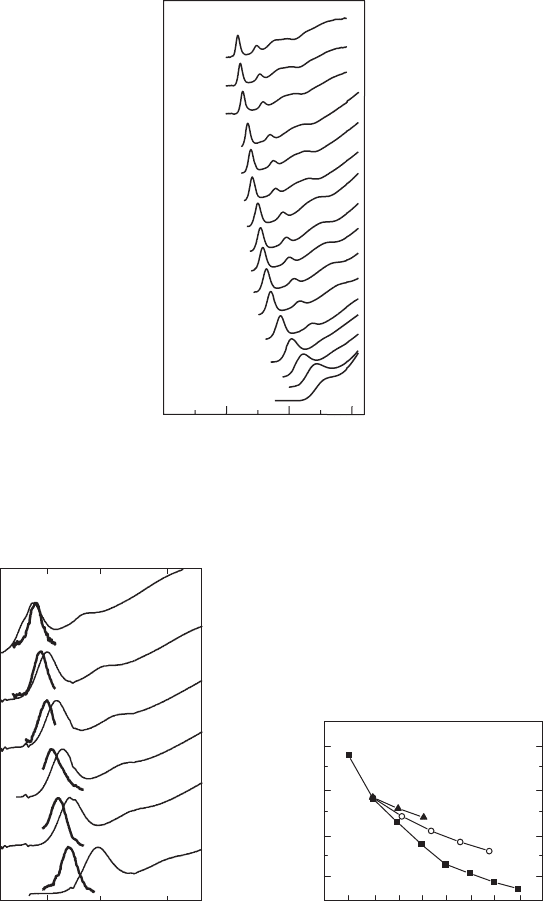
The room temperature absorption spectra of PbSe NQD core samples, with average NQD diam-
eter varying from 2.8 nm to 8 nm, are shown in Fig. 25.4 , showing a strong blue shift of the
absorption edge with a decrease of the NQDs ’ size. The top four curves correspond to the samples
prepared by a synthetic alternative given in section 25.2.2 producing high-quality NQDs with an
absorption edge around 2 micron. The other absorption curves in Fig. 25.4 correspond to NQDs
that were prepared by the synthesis described in section 25.2.1. It should be mentioned that the
absorbance and PL spectra of the PbSe NQDs, stabilized by the AET water-soluble ligands, were
identical to the corresponding NQDs capped with organic ligands, before the ligand exchange.
These observations were discussed in details in [27] , leading to a conclusion that the ligand
exchange at the NQDs ’ surfaces did not alter the size and the quality of the NQDs.
Comparison between the absorption (thin lines) and the PL (bold lines) spectra of PbSe NQDs,
with various diameters and capped with organic ligands, is shown in Fig. 25.5a . A plot of the 1S
(a)
(b)
Figure 25.3 TEM images of PbSe NQDs. (a) Capped with OA/TOP ligands in an organic medium. (b) Capped with
the AET ligand in an aqueous medium. The insets in panels (a) and (b) represent the corresponding electron diffrac-
tion patterns.
CH025-I046325.indd 755CH025-I046325.indd 755 6/25/2008 11:16:20 AM6/25/2008 11:16:20 AM
756 Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics
exciton absorption energy versus NQD size is plotted in Fig. 25.5b (line 1). The PL curves shown
in Fig. 25.5a exhibit an energy Stokes shift with respect to the lowest exciton absorption band,
which is reduced gradually from ⬃100 meV for NQDs with a 4.0 nm diameter, to an anti-Stokes
shift of 10 meV for NQDs with a diameter of 6.1 nm.
A rock salt PbSe semiconductor exhibits direct band transitions at the L-point of the Brillouin
zone, with four-fold degeneracy. As mentioned in the Introduction, currently there are three
0.0
1.0
1.5
8.0 nm
7.0 nm
6.0 nm
4.4 nm
2.3 nm
Energy (eV)
Absorbance (a.u.)
0.5
Figure 25.4 Absorption spectra of PbSe NQD core samples, with average NQD diameters varying from 2.8 nm to
8 nm. The top four curves correspond to the samples prepared by a synthetic alternative given in section 25.2.2. The
other absorption curves correspond to NQDs that were prepared by the synthesis described in section 25.2.1.
0.8 1.2
(a)
6.1 nm
5.6 nm
5.3 nm
5.1 nm
5.0 nm
3.7 nm
Absorbance (a.u.)
PL (a.u.)
Energy (eV)
246810
0.6
0.8
1.0
1.2
3
2
1
1S exciton
absorption energy
Diameter of NQDs (nm) (b)
Figure 25.5 (a) Absorption (thin lines) and PL (bold lines) spectra of PbSe NQDs with various average diameters.
(b) Plot (1) of the 1S exciton absorption energy versus the diameter for PbSe core NQDs, (2) of PbSe/PbS core–
shell NQDs with a PbSe core diameter of 4.2 nm and n - MLs ( n 1–4) of PbS shell, and (3) of PbSe/PbSe
x
S
1
x
core-alloyed–shell NQDs with a PbSe core diameter of 4.2 nm and an increased sulphur percentage (1 x 0.5).
CH025-I046325.indd 756CH025-I046325.indd 756 6/25/2008 11:16:21 AM6/25/2008 11:16:21 AM
PbSe Core, PbSe/PbS and PbSe/PbSe
x
S
1 x
Core–Shell Nanocrystal Quantum Dots 757
main treatments of the electronic confi guration of PbSe NQDs, based either on an effective
mass approximation [2–4] , tight binding model [5] or atomistic pseudo-potential method [6] .
According to the effective mass approximation, ignoring the L-point lift of degeneracy, the
three observed absorption bands, quoted according to an increasing energy order, correspond
to the allowed 1S
h
–1S
e
, the forbidden 1S
h
–1P
e
or 1P
h
–1S
e
and the allowed 1P
h
–1P
e
transitions.
According to the tight binding model and to the pseudo-potential method the second absorp-
tion band corresponds to the 1P
h
–1P
e
allowed transition, about the L– Γ line of the Brillouin
zone, while the third absorption band corresponds to the allowed 1D
h
–1D
e
transition. Recent,
resonance-tunnelling spectroscopy measurements carried out by Liljeroth et al. [31] showed an
agreement with the tight binding model.
The room temperature absorption (thin lines) spectra and the PL (bold lines) spectra of the
PbSe/PbS core–shell NQDs, with a 4.9 nm PbSe core, and n -MLs of PbS shell ( n 0, 1, 2, 3),
are presented in Fig. 25.6a . These spectra exhibit a systematic red shift of the absorption as well
as the emission bands, with the increase of the shell thickness, when compared with the cor-
responding core sample. A plot of the 1S exciton absorption energy versus the overall diameter
of the PbSe/PbS core–shell NQD is shown in Fig. 25.5b (line 2). It is interesting to note that
the full width at half maximum (FWHM) of the PbSe/PbS core–shell NQDs 1S emission bands
(⬃50 meV) is smaller than that of the PbSe corresponding core samples (⬃65 meV). In addition,
the PL band of PbSe/PbS core–shell NQDs with one PbS ML is red shifted with respect to the fi rst
absorption band by 30 meV (Stokes shift), while the PL band of the NQDs sample with three PbS
MLs is blue shifted with respect to the fi rst absorption band by 10 meV (anti-Stokes shift).
0.8 1.2
(a) (b)
3 ML
2 ML
1 ML
Core
Absorbance (eV)
PL (a.u.)
Energy (eV)
0.6 0.8 1.0 1.2
x 0.5
x 1
Absorbance (a.u.)
PL (a.u.)
Energy (eV)
Figure 25.6 (a) Representative absorption (thin lines) and PL (bold lines) spectra of PbSe/PbS core–shell NQDs
with a PbSe core diameter of 4.9 nm and an increased number of PbS shell MLs, as indicated in the fi gure. (b)
Absorption (thin lines) and PL (bold lines) spectra of PbSe/PbSe
x
S
1
x
core-alloyed shell NQDs with x varying from
x 1 to x 0.5.
The absorption and PL spectra of the PbSe/PbSe
x
S
1
x
core-alloyed–shell NQDs prepared with
various stoichiometric compositions are shown in Fig. 25.6b (thin and bold lines, respectively).
The PL spectra of the PbSe/PbSe
x
S
1
x
NQDs exhibit a peculiar behaviour similar to those dis-
cussed in the preceding paragraph, showing a large (up to 50 meV) anti-Stokes shift with
respect to the fi rst absorption band for a shell composition of PbSe
x
S
1
x
with x 0.5. Also, the
PL QE of the PbSe/PbSe
x
S
1
x
( x 0.5) core-alloyed–shell samples was measured to be 65%,
CH025-I046325.indd 757CH025-I046325.indd 757 6/25/2008 11:16:22 AM6/25/2008 11:16:22 AM
758 Handbook of Self Assembled Semiconductor Nanostructures for Novel Devices in Photonics and Electronics
higher than the corresponding PL QE of PbSe core and PbSe/PbS core–shell samples, which were
measured between 40% and 50%. Again, there is a red shift of the absorption spectra with the
increase of the sulphur percentage in the shell composition, as shown in Fig. 25.5b (line 3).
The absorption and emission spectra shown so far suggest that a minor anti-Stokes shift exists
already in the core samples. However, this peculiar behaviour is more pronounced in the core-
alloyed–shell samples ( Fig. 25.6b ). Moreover, it should be remembered that the entire spectrum
of the core–shell samples is red shifted in comparison with that of the corresponding core NQDs.
The red shift of the absorbance and PL bands of the PbSe/PbS core–shell samples and the PbSe/
PbSe
x
S
1
x
core-alloyed–shell samples (top curves in Fig. 25.6 ), with respect to that of a reference
core PbSe sample (bottom curves in Fig. 25.6 ) can be explained by an anomalous sequence of
the valence and conduction band edge energies, when E
c
(PbS) E
c
(PbSe) E
v
(PbS) E
v
(PbSe),
as reported in bulk IV–VI compounds [32] . Assuming that such an anomalous behaviour is
retained in the nano-size, a spread of the hole wavefunction over E
V
(PbSe) and E
v
(PbS) states
might occur, leading to a red shift of the optical transitions with respect to the optical transitions
of the core. Furthermore, the existence of an alloyed shell leads to tunability of the energy dif-
ference Δ E E
v
(PbSe)– E
v
(PbSe
x
S
1
x
), depending on the shell composition ( x ) and on the shell
thickness. It should be mentioned that expansion of a wavefunction over the core–shell interface
was also observed in CdSe/ZnS and InP/ZnS core–shell NQDs, with an exceptionally large poten-
tial barrier induced by the ZnS shell [33–37] . Also, Wise et al. [38] estimated recently a theo-
retical model, describing the electron–hole wavefunctions in PbSe/PbS core–shell NQDs. They
proposed the existence of a type I NQD with a spreading of both electron and hole wavefunctions
over the entire NQDs to samples with a core diameter of 3 nm and a shell thickness of 1.7 nm.
When the shell thickness becomes larger than 2.5 nm, then a separation of the electron–hole
wavefunctions becomes more pronounced, suggesting a type II confi guration. In addition, when
the core size becomes larger than 6.0 nm, the wavefunction overlap is reduced. The agreements
of those calculations with the experimental data are currently being investigated. In general,
the larger the separation of the electron–hole wavefunction (approaching type II confi guration)
the longer the recombination lifetime would be, in agreement with our preliminary results indi-
cated above.
The red shift of the PL bands with respect to their corresponding 1S exciton absorption energy
is also an issue of major concern. As mentioned above, the PbSe electronic structure consists of
valence and conduction band edges at the L-point of the Brillouin zone with a four-fold degen-
eracy, which is doubled by the spin states. Therefore, the interband transitions may involve 8 8
possible transitions (involving the bright and dark excitons). Indeed, Delerue et al. [5] and Zunger
et al. [6] recently discussed a breaking of the degeneracy due to occurrence of intervalley inter-
actions as well as the existence of strong anisotropy of the band edge states with different values
for the longitudinal (m||) and transverse (m ⬜ ) effective masses. Thus, the PbSe NQD excitation
may occur into a bright state (1 out of the 64 possibilities) and emission will take place from a
lower energy bright or dark state. In addition, the energy manifold of exciton states varies with
the change of the NQDs ’ diameter, leading to a gradual change in the Stokes shift.
The appearance of an anti-Stokes shift is a matter of debate. As mentioned above, it does exist
in the PbSe core samples ( 10 meV) and it is substantially more pronounced in the PbSe/PbS
core–shell and PbSe/PbSe
x
S
1
x
core-alloyed–shell structures (up to 50 meV). Careful obser-
vation of Figs. 25.5a and 25.6a and b reveals that the anti-Stokes shift becomes pronounced
around 0.77 eV, a point were the energy shift of the emission band versus the NQDs ’ size nearly
riches a plateau, while that of the absorption 1S exciton still continues. Currently, we presume
that the peculiar appearance of the anti-Stokes shift comes about due to the fact that the smaller
NQDs, emitting at the blue side of the band, are having larger emission oscillator strength, as
proposed by Brus et al. [39] and Efros et al. [40] . Even though their volume fraction is not neces-
sarily larger, an enhancement in the luminescence intensity at the high energy side of the emis-
sion band appears, artifi cially shifting the emission Gaussian envelope further to the blue. This
is mainly pronounced in samples in which the exchange interaction is relatively small and the
Stokes shift is reduced (upper curves as the indicated fi gures).
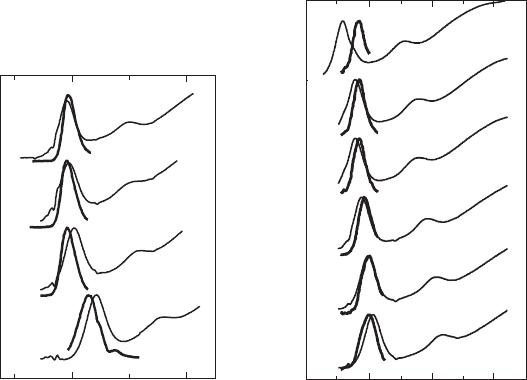
Figure 25.7 shows representative FLN spectra of PbSe core (A) and PbSe/PbS core–shell (B)
NQD samples. These curves are compared with the non-resonance PL spectra (lower curves in
CH025-I046325.indd 758CH025-I046325.indd 758 6/25/2008 11:16:23 AM6/25/2008 11:16:23 AM
