Ghasem D. Najafpour. Biochemical Engineering and Biotechnology
Подождите немного. Документ загружается.

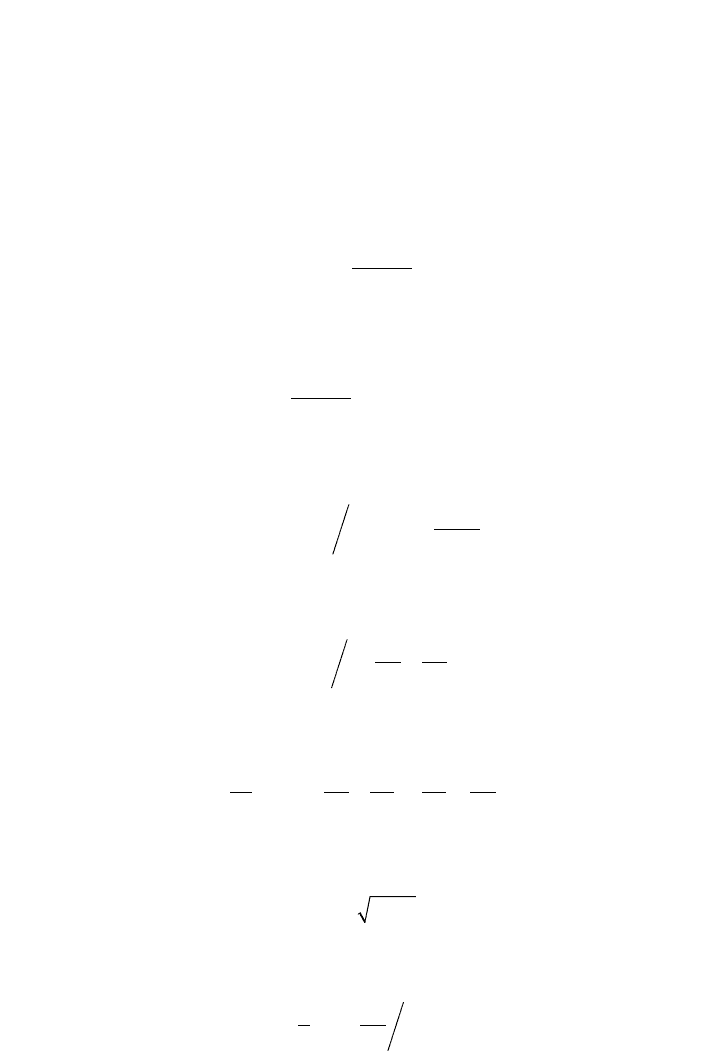
Equation 5.7.1.34 gives:
(5.7.1.36)
Equation 5.7.1.32 leads to:
(5.7.1.37)
The free enzyme concentration is:
(5.7.1.38)
Substituting (5.7.1.38) into (5.7.1.36) results in:
(5.7.1.39)
The intermediate complex ES is defined:
(5.7.1.40)
The enzyme rate equation with two dissociation relations at equilibrium yields:
(5.7.1.41)
Now, maximise the rate at a specific substrate concentration:
(5.7.1.42)
Maximum substrate concentration is defined by the square root of the dissociation constants
(5.7.1.43)
At a high substrate concentration, the rate can be simplified and a linearised model is obtained:
(5.7.1.44)
1
1
2
y
⫽⫹
[]S
K
ke
o
SKK
max
⫽
12
d
d
y
tK
K
S
K
S
K
⫽⫽⫺ ⫹ ⫽0
11
2
1
2
1
2
2
y ⫽⫹⫹ke
S
K
K
S
o
1
2
1
[]
[]
[]
[]
ES K e S K
KK
S
o
⫽⫹⫺
22
21
È
Î
Í
˘
˚
˙
Ke K
KES
S
KES ESS
o22
1
2
⫺⫺⫽
[]
[]
[][][]
[]
[]
[]
E
KES
S
⫽
1
KES ES
1
[][][]⫽
Ke KE K ES ES S
o222
⫺⫺ ⫽[][][]
104 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 104
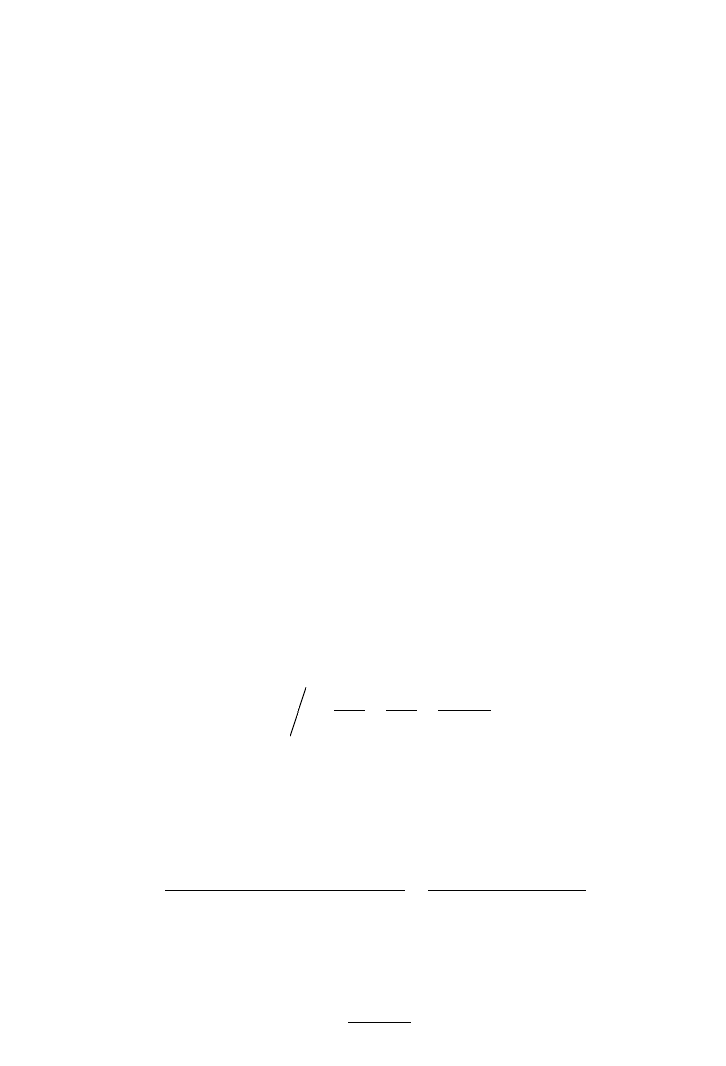
A graph of 1/y versus S is plotted and the slope is 1/K
2
ke
o
. There is an intercept in the
graphical presentation to identify another constant. From the above equation, K
2
can be cal-
culated, which is similar to S
max
, where S
max
means that the substrate concentration gives
the maximum rate.
5.7.2 Kinetics of Reversible Reactions with Dual Substrate Reaction
The reaction mechanisms for reversible reactions are slightly different. In the above sec-
tion, the second part of the reaction that leads to product was irreversible. However, if all
the steps in enzyme reactions were reversible, the resulting rates may be affected.
(5.7.2.1)
where k
1
is the rate constant for forward and k
⫺1
the rate constant for backward reactions.
The second reaction is:
(5.7.2.2)
where k
2
is the rate constant for forward and k
⫺2
the rate constant for backward reactions.
For dual substrates the reaction mechanisms may be complicated if the enzyme–substrate
complex of the first substrate reacts with the second substrate; then the dissociation con-
stant of K
12
is defined to present the equilibrium, and vice versa the dissociation constant
for the reaction of second substrate–enzyme complex with the first substrate is K
21
.
(5.7.2.3)
The rate equation for reversible reactions with two substrates is defined.
(5.7.2.4)
Assume
(5.7.2.5)
(5.7.2.6)
For the special case the rates are simplified to more familiar rates and result in the following:
(5.7.2.7)
y
y
⫽
⫹
max
*
S
KS
1
11
*
y ⫽
⫹⫹⫹
⫽
⫹⫹
ke S S
SS KS KS KK
ke S S
KSKS
oo21
12 212 121 2 21
21
12 2 1 1
()()
*
KK KK
112 2 21
=
y ⫽ ⫹⫹⫹ke
K
S
K
S
KK
SS
o
1
21
1
12
2
221
12
e e ES ES ES S
o
⫽⫹ ⫹ ⫹
1212
ES P E ⫹
SE ES
k
k
⫹
⫺
1
1
¨Æææ
GROWTH KINETICS 105
Ch005.qxd 10/27/2006 10:44 AM Page 105

where y
*
max
and K
1
*
are the apparent maximum rate and Michaelis constant, respectively.
(5.7.2.8)
(5.7.2.9)
If one substrate is in great excess, K
12
is small or S
2
⬎⬎ K
12
then y
max
⫽ ke
o
and K
*
1
⬵K
21
. In
this case we can simplify the rate to:
(5.7.2.10)
5.7.3 Reaction Mechanism with Competitive Inhibition
Generally inhibitors are competitive or non-competitive with substrates. In competitive
inhibition, the interaction of the enzyme with the substrate and competitive inhibitor
instead of the substrate can be analysed with the sequence of reactions taking place; as a
result, a complex of the enzyme–inhibitor (EI) is formed. The reaction sets at equilibrium
and the final step shows the product is formed. The enzyme must get free, but the enzyme
attached to the inhibitor does not have any chance to dissociate from the EI complex. The
EI formed is not available for conversion of substrate; free enzymes are responsible for that
conversion. The presence of inhibitor can cause the reaction rate to be slower than the ordi-
nary reaction, in the absence of the inhibitor. The sequence of reaction mechanisms is:
(5.7.3.1)
(5.7.3.2)
where K
S
and K
i
are dissociations for the Michaelis–Menten rate constant and the inhibi-
tion constant, respectively:
(5.7.3.3)
The total enzyme concentration is the sum of all enzymes as free, and those conjugated as
ES and EI:
(5.7.3.4)
e E ES EI
o
⫽⫹ ⫹
ES E P
k
æÆæ ⫹
EI EIK
i
⫹ ,
ES ESK
S
⫹ ,
y ⫽
⫹
ke S
KS
o 1
21 1
K
KK K S
KS
1
112 122
12 2
*
⫽
⫹
⫹
y
max
*
⫽
⫹
ke S
KS
o 2
12 2
106 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 106
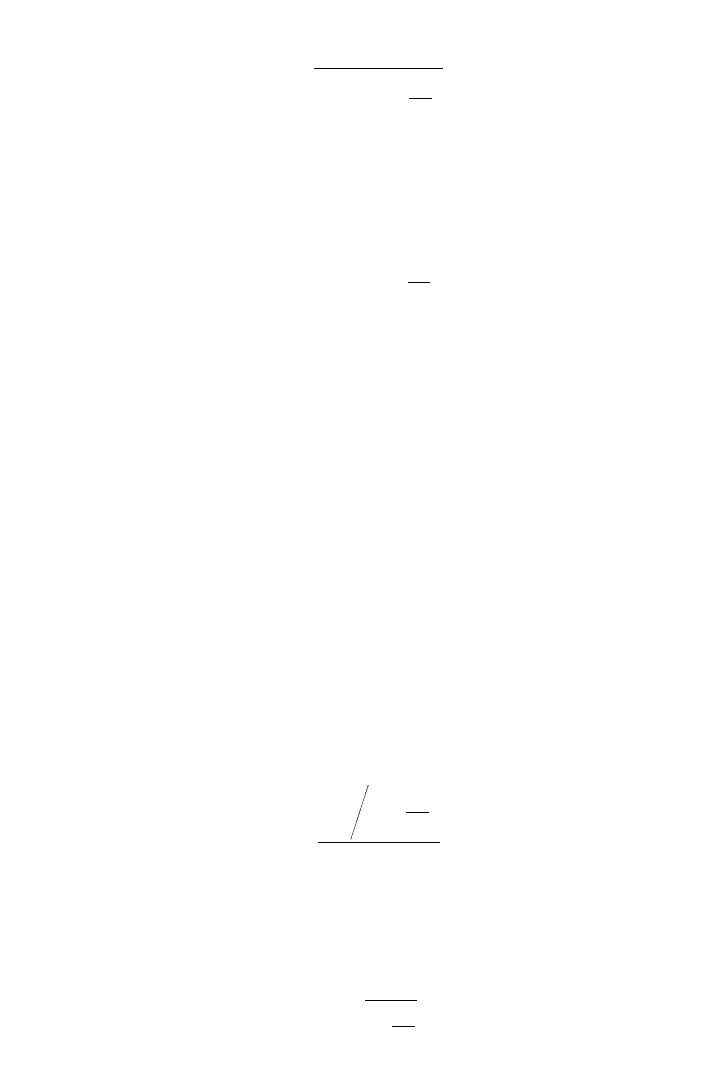
The reaction rate model is based on total enzyme, substrate and inhibitor concentrations.
(5.7.3.5)
Comparison of the ordinary Michaelis–Menten relation with (5.108) shows that the inhibitor
did not influence specific growth rate, y
max
, but the Michaelis–Menten constant was affected
by the inhibitor and resulted in a constant, known as the apparent Michaelis constant.
(5.7.3.6)
where K
app
m
is apparent Michaelis constant. The rate constant is increased by the presence
of a competitive inhibitor. The inhibitor causes the reaction rate to slow down. The compet-
itive inhibitor can be unaffected or eliminated by increasing the substrate concentration.
5.7.4 Non-competitive Inhibition Rate Model
The non-competitive inhibitor is defined by the following sequence of reactions:
(5.7.4.1)
(5.7.4.2)
In such inhibition, the inhibitor and the substrate can simultaneously bind to the enzyme.
The nature of the enzyme–inhibitor–substrate binding has resulted in a ternary complex
defined as EIS. The K
S
and K
i
are identical to the corresponding dissociation constants. It
is also assumed that the EIS does not react further and is unable to deliver any product P.
The rate equation for non-competitive inhibition, u
max
, is influenced:
(5.7.4.3)
The maximum specific growth rate is retarded with non-competitive inhibitor. The appar-
ent specific growth rate, y
app
max
, is smaller than the ordinary specific growth rate, y
max
.
(5.7.4.4)
y
max
app
⫽
⫹
ke
i
K
o
i
1
y ⫽
⫹
⫹
ke S
i
K
SK
o
i
S
1
Ê
Ë
Á
ˆ
¯
˜
ES I ESI K
i
⫹ ,
EI S EIS K
S
⫹ ,
KK
i
K
mS
i
app
⫽⫹1
Ê
Ë
Á
ˆ
¯
˜
y ⫽
⫹⫹
ke S
SK
I
K
o
S
i
1
Ê
Ë
Á
ˆ
¯
˜
GROWTH KINETICS 107
Ch005.qxd 10/27/2006 10:44 AM Page 107

If the complex of ESI can be dissociated to product, the rate equation would result in mixed
competitive and non-competitive inhibitors:
(5.7.4.5)
(5.7.4.6)
The competitive and non-competitive inhibitors are easily distinguished in a Lineweaver–
Burk plot. The competitive inhibitor intercepts on the 1/y axis whereas the non-competitive
inhibitor intercepts on the 1/S axis. The reaction of inhibitors with substrate can be assumed
as a parallel reaction while the undesired product is formed along with desired product. The
reactions are shown as:
(5.7.4.7)
(5.7.4.8)
Since enzyme is not shown in the reaction we assume an elementary rate equation may
explain the above reactions. The simple kinetics are discussed in most fermentation tech-
nology and chemical reaction engineering textbooks.
8–10
Example 1
An enzyme is produced for manufacturing a sun protection lotion. Given kinetic data for
the enzyme reaction with , Km ⫽ 8.9 mM and S
O
⫽ 12 mM, what would be
the time required for 95% conversion in a batch bioreactor?
Solution
Example 2
Calculate K
m
and V
max
for given substrate concentrations and rates. The inverse rate and
substrate concentrations are calculated in Table E.2.1.
t
batch
mM
mM/h
h⫽⫹⫽
89
9
12
06
095 12
9
423
.
ln
.
(. )( )
.
V
max
.⫽⫽25
3600 1
1000
9
3
mmol
ms
s
h
m
L
mmol
hL
3
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
v
max
.⫽ 25
mmol
ms
3
AS
k
2
æÆæ undesired
AR
k
1
æÆæ desired
y
y
⫽
⫹
max
app
app
S
SK
m
EIS EI P
k
æÆæ ⫹
108 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 108
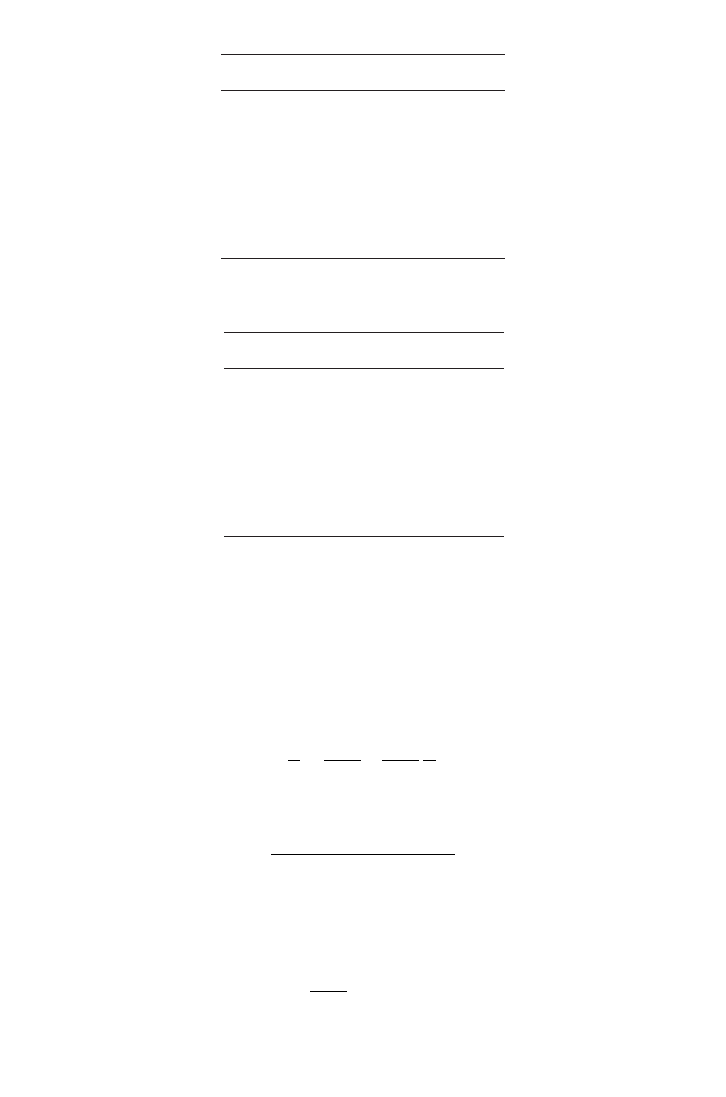
Solution
Let us inverse the substrate concentration and reaction rate as shown in Table E.2.2.
In evaluation of kinetic parameters, the double reciprocal method is used for linearisa-
tion of the Michaelis–Menten equation (5.7.3).
(a) Use the Lineweaver–Burk plot as defined in the following relation:
(E.2.1)
(E.2.2)
K
m
⫽⫻
⫺
9.6 10 mol/l
5
K
m
y
max
.⫽ 0 077
y
max
4
1.25 10 mol/l min⫽⫻
⫺
⭈
Slope ⫽
⫺
⫻⫺⫻
⫽
22500 10000
1 875 10 2 5 10
0 077
54
..
.
11 1
yy y
⫽⫹
max max
K
S
m
GROWTH KINETICS 109
TABLE E.2.1. Substrate concentration
and reaction rate
S (mol⭈l
⫺1
) y (mol⭈l
⫺1
⭈min
⫺1
)
4.10 ⫻ 10
⫺3
1.77 ⫻ 10
⫺4
9.50 ⫻ 10
⫺4
1.73 ⫻ 10
⫺4
5.20 ⫻ 10
⫺4
1.25 ⫻ 10
⫺4
1.03 ⫻ 10
⫺4
1.06 ⫻ 10
⫺4
4.90 ⫻ 10
⫺5
8.00 ⫻ 10
⫺5
1.06 ⫻ 10
⫺5
6.70 ⫻ 10
⫺5
5.10 ⫻ 10
⫺6
4.30 ⫻ 10
⫺5
TABLE
E.2.2.
1/S,l⭈mol
⫺1
1/y,l⭈min⭈mol
⫺1
243.9 5650
1052.6 5780
1923.0 8000
9708.7 9434
20408.1 12500
94339.6 14925
196078.4 23256
Ch005.qxd 10/27/2006 10:44 AM Page 109
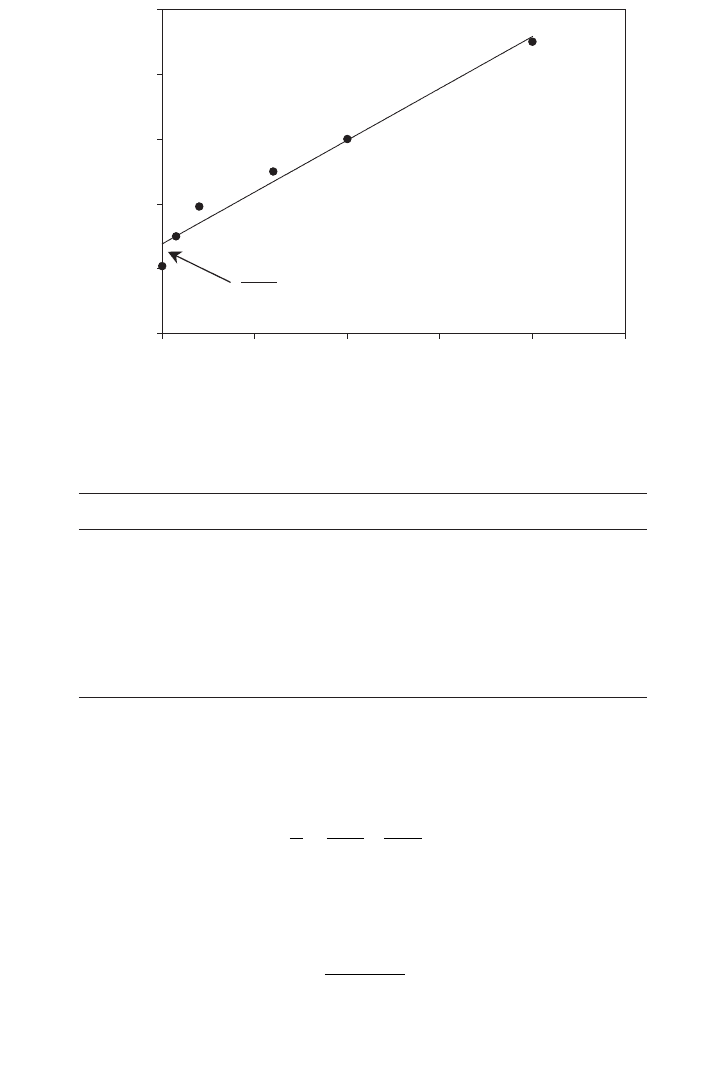
(b) Use another form of linear graphical presentation to evaluate K
m
and V
max
based on
the following relation:
(E.2.3)
This method tends to create a cluster of data near the origin as shown in Figure E.2.2.
y
max
.⫽⫻
⫺
182 10
4
mol/l min⭈
Slope ⫽
⫺
⫻
⫽
⫺
22 5 6 0
310
5500
3
..
S
K
S
m
yy y
⫽⫹
max max
1
110 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
1/s, l/mol
0.0 5.0e+4 1.0e+5 1.5e+5 2.0e+5 2.5e+5
1/ν, l. min. mol
-1
0
5000
10000
15000
20000
25000
max
1
FIG. E.2.1. Lineweaver–Burk plot.
TABLE
E.2.3. Data collected and calculated for rate model
S (mol⭈l
⫺1
) y, mol⭈l
⫺1
⭈min
⫺1
S/y,min y/S,min
⫺1
4.10 ⫻ 10
⫺3
1.77 ⫻ 10
⫺4
23.00 0.044
9.50 ⫻ 10
⫺4
1.73 ⫻ 10
⫺4
5.50 0.182
5.20 ⫻ 10
⫺4
1.25 ⫻ 10
⫺4
4.20 0.240
1.03 ⫻ 10
⫺4
1.06 ⫻ 10
⫺4
0.97 1.030
4.90 ⫻ 10
⫺5
8.00 ⫻ 10
⫺5
0.60 1.670
1.06 ⫻ 10
⫺5
6.70 ⫻ 10
⫺5
0.16 6.250
5.10 ⫻ 10
⫺6
4.30 ⫻ 10
⫺5
0.12 8.330
Ch005.qxd 10/27/2006 10:44 AM Page 110
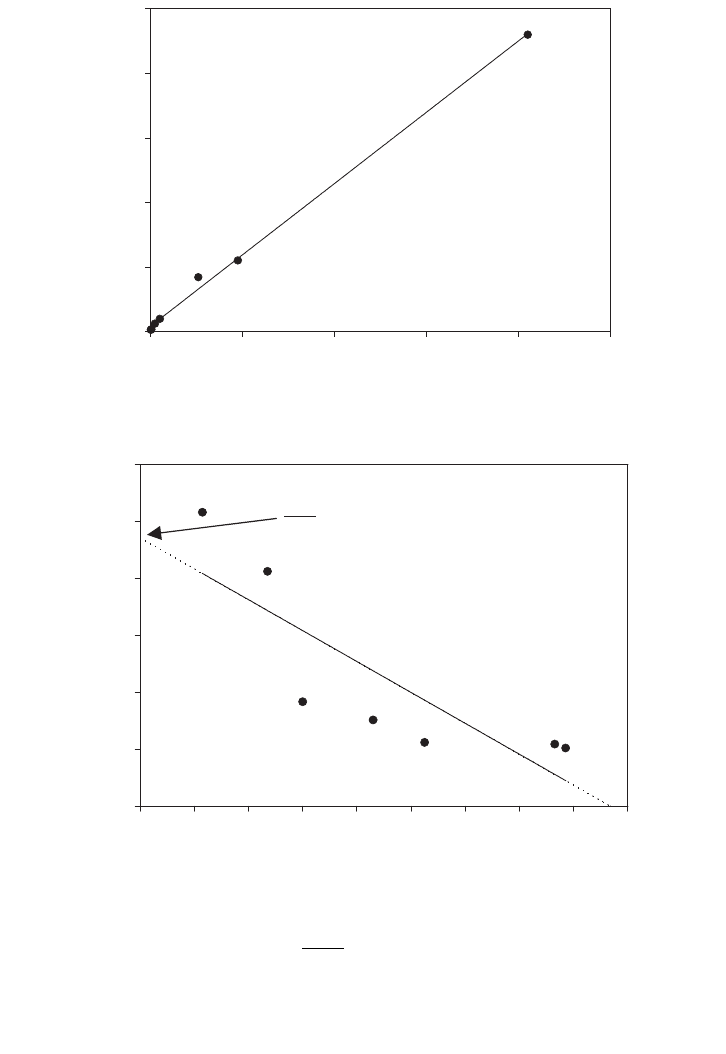
For the Eadie–Hofstee plot, both coordinates contain rates that are subjected to the great-
est error, as indicated in Figure E.2.3.
K
m
⫽⫽⫻
⫺
05
5500
91 10
5
.
.mo
GROWTH KINETICS 111
S, mol/l
0.000 0.001 0.002 0.003 0.004 0.005
S/ν, min
0
5
10
15
20
25
FIG. E.2.2. Linear model for the Monod rate equation with populated data at the origin.
ν, mol.l
-1
.min
-1
2e-5 4e-5 6e-5 8e-5 1e-4 1e-4 1e-4 2e-4 2e-4 2e-4
ν/S, min
-1
-2
0
2
4
6
8
10
K
m
max
FIG. E.2.3. Eadie–Hofstee plot.
Ch005.qxd 10/27/2006 10:44 AM Page 111
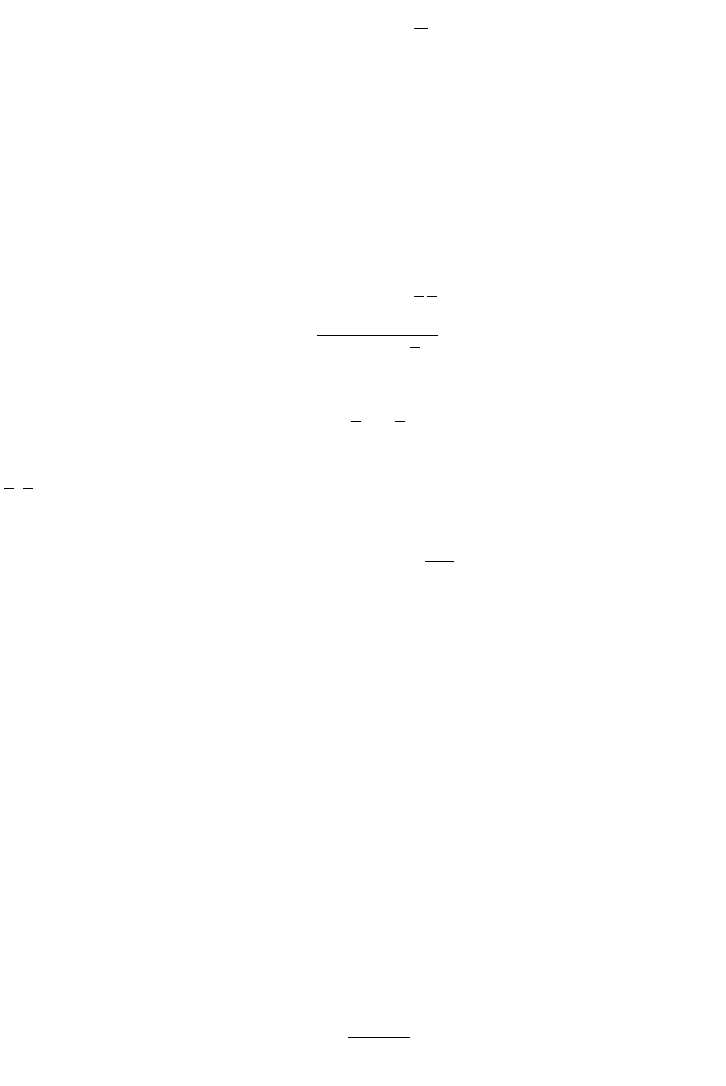
(E.2.3)
A plot of u/S versus y is presented in Figure E.2.3 for defining slope and intercept, K
m
and
y
max
, respectively.
A linearisation model is used to explain the equation of a simple straight line:
9
(E.2.4)
where
(E.2.5)
(E.2.6)
are average values of x
i
and y
i
Example 3
In batch enzyme reaction kinetics, given K
m
⫽ 10
⫺3
M and substrate concentration S
0
⫽
3 ⫻ 10
⫺5
M, after 2 min, 5% of the substrate was converted. How much of the substrate was
consumed after 10, 20, 30 and 60 min?
Solution
For S
o
⬍⬍ K
m
, the rate model is reduced to a first-order rate equation:
The Michaelis–Menten rate equation is:
(E.3.1)
y
y
⫽
⫹
max
S
KS
m
y
max
min⫽⫻
⫺⫺⫺
18 10
411
mol l⭈⭈
K
m
⫽⫻
⫺⫺
65 10
51
. mol l⭈
Slope of the line ⫽
1
K
m
aybx⫽⫺
b
xy Nxy
xNx
ii
i
N
i
⫽
⫺
⫺
Â
Â
22
ybxa⫽⫹
yy
y
⫽⫺
max
K
S
m
112 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
xy,
Ch005.qxd 10/27/2006 10:44 AM Page 112

The simplified first-order rate is
(E.3.2)
Carry out integrations:
(E.3.3)
(E.3.4)
The concentration profile is predicted by the following equation
(E.3.5)
At time equal to 2 min:
(E.3.6)
(E.3.7)
(E.3.8)
(E.3.9)
(E.3.10)
v
S
S
m
o
t
⫽⫺
⫽
⫽
⫺
⫺
ln .
. min
.
095
2
0 2565
1
0 02565
e
e
⫺
⫽
2V
m
*
.095
S
S
o
⫽ 095.
CC X
AA A
o
⫽⫺()1
S
S
X
o
E
⫽⫺1
SSe
Vt
m
⫽
⫺
0
*
ln
S
S
t
o
m
⫽⫺n
⫺⫽
d
d
S
S
Vt
m
t
S
S
o
*
0
ÚÚ
⫺⫽
d
d
S
t
VS
m
*
GROWTH KINETICS 113
Ch005.qxd 10/27/2006 10:44 AM Page 113
