Ghasem D. Najafpour. Biochemical Engineering and Biotechnology
Подождите немного. Документ загружается.

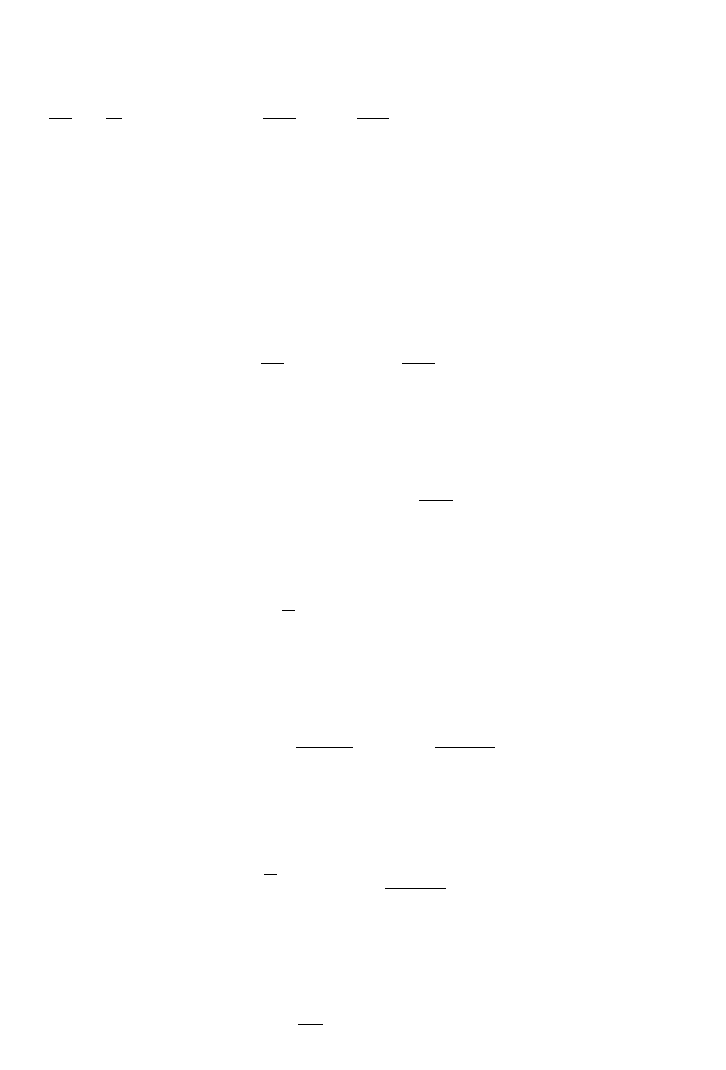
5.6.7 Material Balance in Terms of Substrate in a Chemostat
The substrate balance is given based on following equation:
(5.6.7.1)
In general, m ⬎⬎ m, so we can therefore neglect the last term.
For steady-state no product is formed which means there are no changes in substrate and
product concentrations:
(5.6.7.2)
Also, F/V ⫽ D then m ⫽ D
(5.6.7.3)
Rearranging the above equation:
(5.6.7.4)
For steady-state condition:
(5.6.7.5)
Substituting equation (5.6.7.2) into equation (5.6.7.1):
(5.6.7.6)
For unsteady state:
(5.6.7.7)
d
d
X
t
XD⫽⫺()m
XY S
KD
D
XS
S
m
=-
-
Ê
Ë
Á
ˆ
¯
˜
/in
m
D
S
KS
S
KD
D
m
S
S
m
⫽
⫹
⫽
⫺
m
m
out
XY S S
XS
⫽⫺
/
()
in out
0 ⫽⫺⫺DS S
X
Y
XS
()
/
in out
m
⫺⫽ ⫽
d
d
and
S
t
q
Y
P
PS
00
/
Ê
Ë
Á
ˆ
¯
˜
⫺⫽ ⫺ ⫺ ⫺ ⫺
d
d
cell
in out
S
t
F
V
SS
Y
X
q
Y
XmX
XS
P
PS
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
()
//
m
pproduct Maintenance 0
growing formed
⫽
94 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 94
The equation with respect to substrate:
(5.6.7.8)
5.6.8 Modified Chemostat
With cell recycling, chemostat efficiency is improved. To maintain a high cell density the
cells in the outlet stream are recycled back to the fermentation vessel. Figure 5.10 repre-
sents a chemostat unit with a cell harvesting system. The separation unit is used for har-
vesting the cells and recycling then to the culture vessel to increase the cell concentration.
The material balance in a constant volume chemostat is derived based on cell balance as
shown in the following equations. Material balance in a chemostat with recycle, r
cell
:
(5.6.8.1)
where t ⫽ recycle ratio
c ⫽ the factor by which the outlet stream is concentrated before return
d
d
X
t
F
V
XX X
F
V
cX
o
⫽⫺⫹⫹⫹
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
[()]1 tm ⭈
d
d
X
t
X
S
KS
D
m
S
⫽
⫹
⫺m ⭈
Ê
Ë
Á
ˆ
¯
˜
GROWTH KINETICS 95
(1+)F, X
Cell separator
FS
r
F
F
Concentrated cells, CX
Effluent
Cotton
filter
Medium in
Cotton
filter
FIG. 5.10. Chemostat with a cell recycle stream.
Ch005.qxd 10/27/2006 10:44 AM Page 95
For steady-state,
(5.6.8.2)
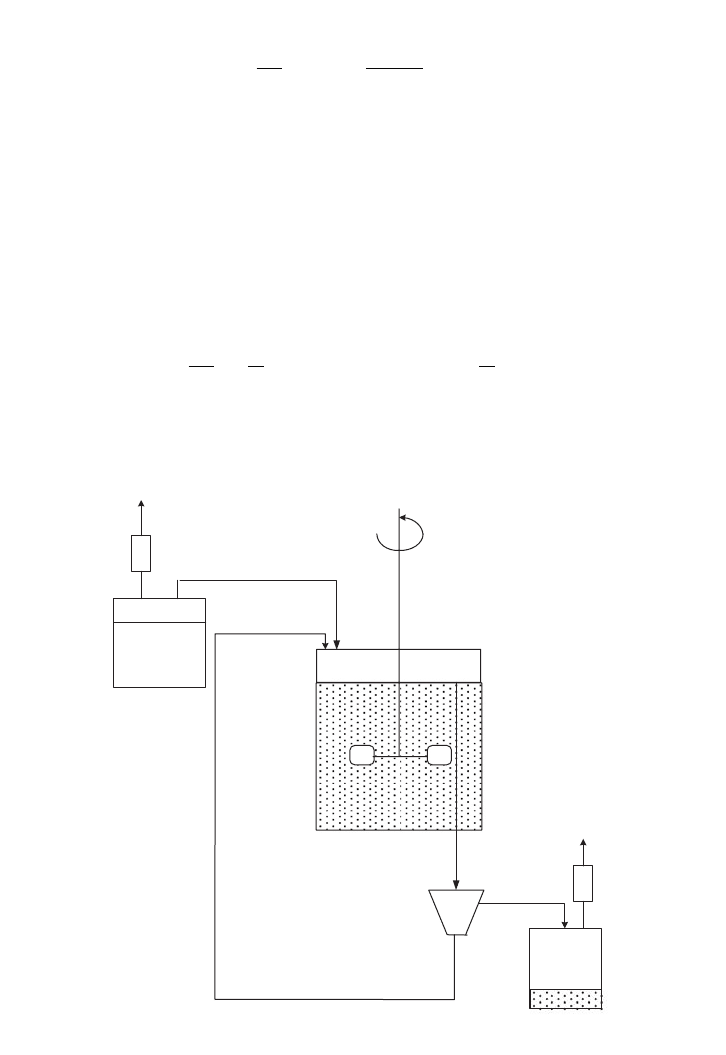
Multi-stages of continuous culture are designed to use the outlet of the first vessel as the
inoculum for the next stage. If intermediate metabolites are used as feed for another
microorganism, sequential continuous culture is useful. The dilution rate for each vessel
may be different to the other vessel. It is also possible to supply different nutrients for each
stage of fermentation vessel. It is common to operate earlier stages as aerobic and subse-
quent stages in an anaerobic condition. In addition, if unused substrate leaves the product
stream, it can be used in the next stage even at low substrate concentration. The kinetic rep-
resentation may show a slower rate and even drop to zero-order. Figure 5.11 shows two
stages of a chemostat in operation.
5.6.9 Fed Batch Culture
Culture with continuous nutrient supply can be operated in two modes: (i) variable volume;
(ii) fixed volume.
For variable volume, feed rate F
in
is not the same as outflow when F
out
⫽ 0
(5.6.9.1)
d
d
out
t
XV XV F X()⫽⫺m
mtt⫽⫹⫺Dc()1
d
d
X
t
⫽ 0
96 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
S
R
, F
X, S, F
S` F`
X, S, V
X
2
, S
2
, V
2
X
2
, S
2
, F + F`
Medium out
Air
filter
Medium in
Air
filter
FIG. 5.11. Two stages of CSTR fermentation vessels in series.
Ch005.qxd 10/27/2006 10:44 AM Page 96

(5.6.9.2)
and dilution rate,
(5.6.9.3)
Material balance for substrate:
(5.6.9.4)
For steady-state,
(5.6.9.5)
Substituting m:
(5.6.9.6)
(5.6.9.7)
For quasi-steady state, the specific growth rate reaches the media dilution rate, m ⬇ D. If
F
in
⬎ F
out
, the specific growth rate may decrease.
• Fed batch is used to overcome substrate limitations, especially for the production of
antibiotics.
• Avoid substrate inhibition, which can allow a periodic shift of the growth rate.
5.7 ENZYME REACTION KINETICS
Most enzymes catalyse reactions and follow Michaelis–Menten kinetics. The rate can be
described on the basis of the concentration of the substrate and the enzymes. For a single
enzyme and single substrate, the rate equation is:
(5.7.1)
ES ES⫹
d
d
in
X
t
F
V
XDX⫽⫺ ⫽⫺mm
Ê
Ë
Á
ˆ
¯
˜
()
XY S S c
XS i o
⫽⫺⫹⫺
/
()/( )1 tt
X
D
YSS
XS i o
⫽⫺
m
⭈
/
()
d
d
S
t
⫽ 0
d
d
[]
S
t
F
V
SSS
Y
S
ioo
XS
o
⫽⫺⫹⫹⫺()
/
1 t
m
⭈
D
F
V
⫽
in
d
d
in out
V
t
FF⫽⫺
GROWTH KINETICS 97
Ch005.qxd 10/27/2006 10:44 AM Page 97
(5.7.2)
(5.7.3)
(5.7.4)
where V is rate of substrate consumption in mol⭈l
⫺1
⭈h
⫺1
, V
max
is the maximum specific
growth rate in h
⫺1
, S is substrate concentration in g⭈l
⫺1
and K
M
is Michaelis–Menten con-
stant in g⭈l
⫺1
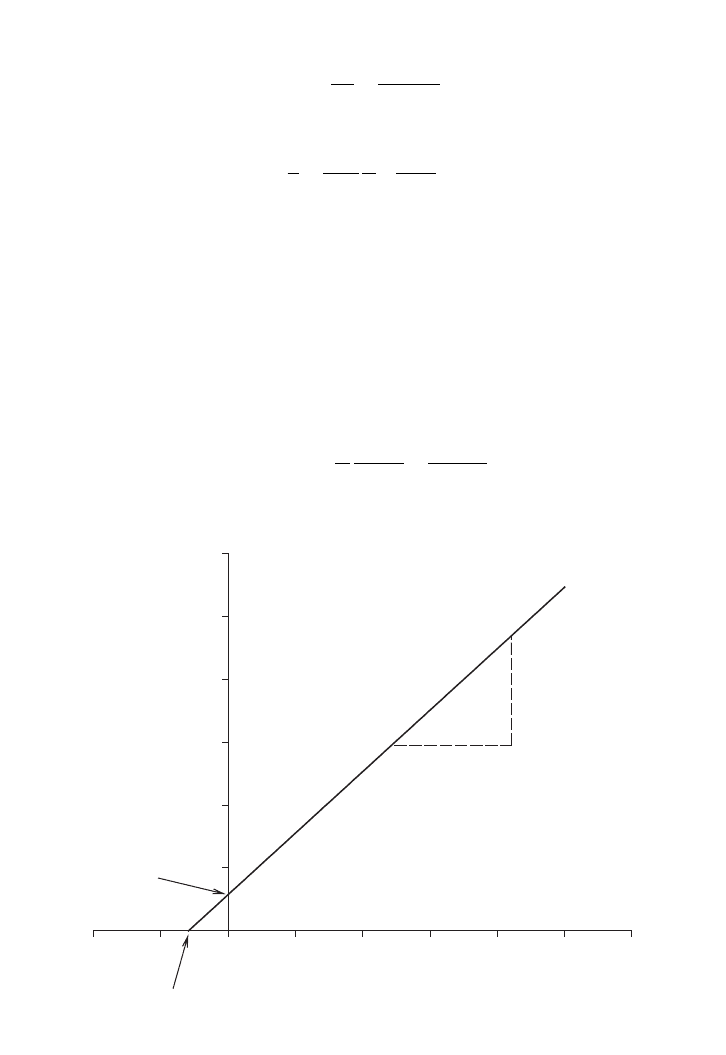
. A double reciprocal plot or a well know Lineweaver–Burk plot of 1/m versus
1/S is shown in Figure 5.12.
For batch reaction, there is no inlet or outlet stream
(5.7.5)
(5.7.6)
⫺⫽⫺⫽ ⫽
⫺
⫹
rr
V
SV
vS
KS
As
m
1d
d
()
max
t
mm
io
⫽⫽0
111
v
K
vS
M
⫽⫹
max max
m
v
S
t
vS
KS
M
⫽⫺ ⫽
⫹
d
d
max
ES E P ⫹
98 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
1/
S
, l/mol
10 -5 0 5 10 15 20 25 30
1/
m
, l. h. mol
-1
0
5
10
15
20
25
30
slope =
K
M
/
m
1/
m
-1/K
M
FIG. 5.12. Single enzyme with different substrates.
Ch005.qxd 10/27/2006 10:44 AM Page 98

where V is the volume of batch reactor which is constant volume.
(5.7.7)
(5.7.8)
(5.7.9)
(5.7.10)
where S
0
is the initial substrate concentration in g⭈l
⫺1
, and S
f
is the final substrate concen-
tration in g⭈l
⫺1
.
5.7.1 Mechanisms of Single Enzyme with Dual Substrates
The kinetics of double substrates with defined dissociation constants are given as:
(5.7.1.1)
where K
1
is the equilibrium or dissociation constant.
(5.7.1.2)
Similarly, for a second substrate, the reaction is carried out and the second product is
formed.
(5.7.1.3)
where K
2
is the equilibrium or dissociation constant.
(5.7.1.4)
ES E P
k
22
2
æÆæ ⫹
ES ESK⫹
222
,
ES E P
k
11
1
æÆæ ⫹
ES ESK
k
k
k
k
⫹⫽
⫺
⫺
111
1
1
1
¨Æææ ,
t
K
v
S
S
SS
v
m
f
f
batch
⫽⫹
⫺
max max
ln
()
0
0
⫺⫽ ⫹ ⫺t
K
v
S
Sv
SS
m
f
o
fo
max max
ln ( )
1
⫺⫽
⫹
ddt
KS
vS
s
t
m
S
S
O
f
0
ÚÚ
max
d
d
S
t
vS
KS
m
⫽⫺
⫹
max
GROWTH KINETICS 99
Ch005.qxd 10/27/2006 10:44 AM Page 99
Overall enzyme balance and equilibrium constants are defined for the intermediate sub-
strate and enzyme complex. The total enzyme concentration is the sum of free and conju-
gated enzymes with the substrates.
(5.7.1.5)
The intermediates, complexes of ES
1
and ES
2
, are defined based on equilibrium constants.
(5.7.1.6)
(5.7.1.7)
The initial and total enzyme concentrations are defined based on measurable components
given below:
(5.7.1.8)
The free enzyme can also be defined based on the following equation:
(5.7.1.9)
The rate equation for first substrate:
(5.7.1.10)
The rate is defined with respect to dual substrates:
(5.7.1.11)
The rate equation for the second substrate is:
(5.7.1.12)
⫺⫽⫽ ⫽
d
d
s
t
kES
kES
K
2
222
22
2
y []
[][ ]
y
1
11
1
1
1
2
2
1⫽⫹⫹
ke S
K
S
K
S
K
o
[]
Ê
Ë
Á
ˆ
¯
˜
⫺⫽⫽ ⫽
d
d
s
t
kES
kES
K
1
111
11
1
y []
[][ ]
E
e
S
K
S
K
o
⫽
⫹⫹1
1
1
2
2
Ê
Ë
Á
ˆ
¯
˜
eE
S
K
S
K
o
⫽⫹⫹1
1
1
2
2
Ê
Ë
Á
ˆ
¯
˜
K
ES
ES
ES
ES
K
2
2
2
2
2
2
⫽⫽
[][ ]
[]
[][ ]
K
ES
ES
ES
ES
K
1
1
1
1
1
1
⫽⫽
[][ ] [][ ]
e E ES ES
o
⫽⫹ ⫹
12
100 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 100

The second rate is also defined as follows:
(5.7.1.13)
Overall reaction rates for dual substrates are the sum of the rates of dissociation of two
substrates.
(5.7.1.14)
If one substrate vanishes then the rate is based on the concentration of the total substrate
that is present in the reaction vessel; so if S
2
is zero, then the total substrate concentration
is the concentration of substrate involved in the reaction.
(5.7.1.15)
Otherwise if one of the substrate increases, the other substrate decreases. If S
2
increases
then S
1
has to decrease. The simplified rate, which is very similar to that for a single sub-
strate, is given as follows:
(5.7.1.16)
In general, enzymes are proteins and carry charges; the perfect assumption for enzyme
reactions would be multiple active sites for binding substrates with a strong affinity to hold
on to substrate. In an enzyme mechanism, the second substrate molecule can bind to the
enzyme as well, which is based on the free sites available in the dimensional structure of
the enzyme. Sometimes large amounts of substrate cause the enzyme-catalysed reaction to
diminish; such a phenomenon is known as inhibition. It is good to concentrate on reaction
mechanisms and define how the enzyme reaction may proceed in the presence of two dif-
ferent substrates. The reaction mechanisms with rate constants are defined as:
(5.7.1.17)
The dissociation constant is related to the equilibrium constant, given by
(5.7.1.18)
ES S ESS ES
K
⫹
2
2
¨Æææ or
k
k
k
1
1
1
=
-
ES ES
k
k
⫹
1
1-
¨Æææ
y
T
OT
T
ekS
KS
O
O
⫽
⫹
2
2
y
T
oT
T
ekS
KS
O
O
⫽
⫹
1
1
S
T
O
y
T
T
o
S
t
S
t
S
t
e
kS
K
kS
K
S
K
S
⫽⫺ ⫽⫺ ⫹ ⫽ ⫹ ⫹ ⫹
d
d
d
d
d
d
12
11
1
22
2
1
1
1
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
22
2
K
Ê
Ë
Á
ˆ
¯
˜
u
2
22
2
1
1
2
2
1⫽⫹⫹
ke S
K
S
K
S
K
o
[]
Ê
Ë
Á
ˆ
¯
˜
GROWTH KINETICS 101
Ch005.qxd 10/27/2006 10:44 AM Page 101

(5.7.1.19)
(5.7.1.20)
The total enzyme concentration is the sum of free and conjugated enzymes with substrates.
(5.7.1.21)
When the enzyme–substrate complex is stabilised, it may reach a fixed concentration,
therefore there is no more change in ES:
(5.7.1.22)
The rate equation for the enzyme complex leads to product in (5.7.1.22) is defined as:
(5.7.1.23)
ES
2
was obtained from (5.7.1.19):
(5.7.1.24)
Incorporating (5.7.1.24) into (5.7.1.22), after simplification it is reduced to:
(5.7.1.25)
Substituting (5.7.1.24) into (5.7.1.22), the rate of enzymatic reaction with dual substrates is
obtained:
(5.7.1.26)
The reaction mechanisms may assist us in obtaining a suitable rate equation. Based on the
enzyme reaction mechanism given by (5.7.1.18) for the intermediate enzyme–substrate
complex, the following equations are derived for ES:
(5.7.1.27)
⫺⫽ ⫺ ⫺ ⫹ ⫽
⫺⫺
d
d
()
[ ][] [ ] [ ][] [ ]
ES
t
kES k ES kESS k ES
1112 22
0
y ⫽
⫺
⫺
kkE S
kk
1
12
[][]
()[][][]kkESkES
⫺
⫺⫽
12 1
[]ES
ES S
k
2
2
⫽
[][]
y ⫽ kES[]
⫺⫽ ⫺ ⫹ ⫺ ⫺⫽
⫺
d
d
[]
[][] [ ] [] [][][]
ES
t
kESS kES k ES kES kES
22211
0
e E ES ES
o
⫽⫹ ⫹
12
ES E P
k
æÆæ ⫹
K
k
k
ES S
ES
2
2
22
⫽⫽
⫺
[][]
[]
102 BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY
Ch005.qxd 10/27/2006 10:44 AM Page 102
From the equilibrium constant, the free enzyme concentration must be defined. We know
the total enzyme concentration as the sum of the conjugated enzymes with substrates and
the free enzymes.
(5.7.1.28)
Substituting (5.7.1.28) into (5.7.1.27), then solving for intermediate enzyme–substrate
complex:
(5.7.1.29)
(5.7.1.30)
The enzyme–substrate complex is used by substituting ES into (5.7.1.23):
(5.7.1.31)
At equilibrium conditions the rate constant for (5.7.1.17) is:
(5.7.1.32)
The intermediate enzyme–substrate complex is defined:
(5.7.1.33)
The second equilibrium constant for the (5.7.1.18) is also defined:
(5.7.1.34)
where the total enzyme is e
o
⫽ E ⫹ ES ⫹ ES
2
E ⫽ e
o
⫺ ES ⫺ ES
2
(5.7.1.35)
Let us eliminate ES
2
by substituting ES
2
⫽ e
o
⫺ E ⫺ ES into equation 5.7.1.27
K
ES S
ES
2
2
⫽
[][]
[]
[]
[][]
ES
ES
K
e S ES ES S
K
o
⫽⫽
⫺⫺
1
22
1
K
ES
ES
1
⫽
[][]
[]
y ⫽⫽
⫹⫺ ⫺⫺
kES
ke S
K
K
k
k
k
S
o
[]
[]
[]11
1
2
1
2
1
Ê
Ë
Á
ˆ
¯
˜
È
Î
Í
˘
˚
˙
ke S k k ES
kk
k
kESS
o111
21
2
2
[]( )[] [][]⫽⫹ ⫺
⫺
⫺
⫺
⫺
Ê
Ë
Á
ˆ
¯
˜
È
Î
Í
˘
˚
˙
ke S k k ES k k ES kESS
o1 1 1 2122
0[][]( )[] [ ] [][]⫺⫹ ⫹ ⫺ ⫺ ⫽
⫺⫺
()
e E ES ES E e ES ES
oo
⫽⫹ ⫹ ⫽ ⫺ ⫺
22
GROWTH KINETICS 103
Ch005.qxd 10/27/2006 10:44 AM Page 103
