Gerya T. Introduction to Numerical Geodynamic Modelling
Подождите немного. Документ загружается.

2.1 Density of rocks and minerals. Equations of state 27
(a) (b)
(c) (d)
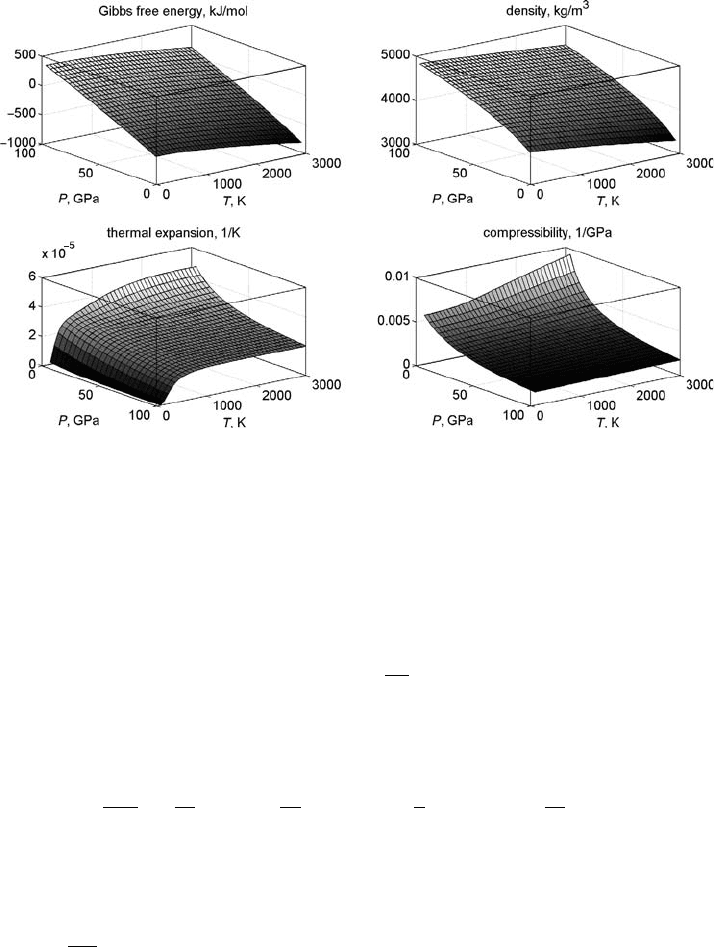
Fig. 2.1 Gibbs free energy (a), density (b), thermal expansion (c) and compress-
ibility (d) of periclase (MgO) computed on the basis of the semi-empirical param-
eterisation of Gerya et al.(2004d) for a broad range of temperature and pressure
values. Note that pressure axis in (c) and (d) is inverted compared to (a) and (b).
Results are computed with the code Periclase_EOS.m.
Murnaghan equation of state (Murnaghan, 1944)
ρ = ρ
0
1 + B
0
P
B
0
1/B
0
, (2.5)
Birch–Murnaghan equation of state (Birch, 1947)
P (ρ) =
3B
0
2
ρ
ρ
0
7/3
−
ρ
ρ
0
5/3
1 +
3
4
B
0
− 4
ρ
ρ
0
2/3
− 1
,
(2.6)
where B
0
=1/β
0
is the bulk modulus (this quantity will be also discussed in
relation to elasticity in Chapter 12) at P = 0(β
0
is the compressibility at P =0),
B
0
=
∂B
∂P
T =const
is the pressure derivative of the bulk modulus at a constant
temperature and ρ
0
is the density at P =0. The Murnaghan equation is derived with
an experimentally based assumption that the pressure derivative of the bulk modulus
B
0
is independent of pressure (many substances have a fairly constant B
0
value of
about 3.5). The Birch–Murnaghan equation (2.6) is also derived empirically based
on measurements of volumes of solid substances, held at a constant temperature.

28 Density and gravity
Obviously, this equation has to be solved iteratively to obtain a density at a given
pressure.
Equations (2.5)and(2.6), however, do not establish the dependence of density
upon temperature, and therefore, an even more complicated EOS must be used
when the density description over a wide range of both pressure and temperature
values is needed. For example, in mineralogy and petrology, the molar volume
of minerals (V) is described in many cases by semi-empirical equations (e.g. the
Murnaghan-like EOS) with additional temperature-dependent terms (Holland and
Powell, 1998)
V = V
r
1 +a
(
T − T
r
)
+ b
√
T −
T
r
×
1 −
B
r
P
B
r
[
1 −c
(
T − T
r
)
]
+ B
r
P
1/B
r
,
(2.7)
where V
r
is molar volume at P
r
and T
r
(i.e.atareference PT-conditions)anda,
b and c are empirical parameters computed from experimental measurements of
molar volume at elevated pressures and temperatures.
It should also be mentioned that in mineralogy and petrology, self-consistent
descriptions of thermodynamic properties (including density) of minerals and flu-
ids is often based on formulating the equations for their molar thermodynamic
potentials (typically either for Gibbs G
(P, T)
or for Helmholz F
(V, T)
potential, e.g.,
Karpov et al., 1976; Helgesson et al., 1978; Dorogokupets and Karpov, 1984;
Berman, 1988; Holland and Powell, 1990, 1998). Density as well as many other
physical properties (such as heat capacity, thermal expansion, compressibility, etc.)
are then computed from the potential (Fig. 2.1) using standard thermodynamic
relations
V =
∂G
(P,T)
∂P
T =cont
, (2.8)
P =−
∂F
(V,T)
∂V
T =cont
, (2.9)
V =
m
ρ
, (2.10)
where m is molar mass, kg/mol.
Self-consistent thermodynamic databases (e.g., Karpov et al., 1976; Helgesson
et al., 1978;Berman,1988; Holland and Powell, 1990, 1998) are often based on
the standard formulation of Gibbs free energy in the form:
G
m(P,T)
= H
r
− T ·S
r
+
T
T
r
[C
Pr(T )
]dT − T ·
T
T
r
[C
Pr(T )
/T ]dT +
P
P
r
[V
(P,T)
]dP,
(2.11)
2.1 Density of rocks and minerals. Equations of state 29
(a)
(b)
2820
2770
2800
2760
2750
2750
2640
2660
2670
2710
2750
2780
2810
2820
2630
2760
2770
2800
2740
2780
2720
350
0.2
0.4
0.6
0.8
0.2
0.4
0.6
0.8
450 550 650 750 850 950
T, °C
350 450 550 650 750 850 950
T, °C
P, GPa
P, GPa
Mu+Chl+Ep
Mu+Chl
Mu+Chl+Bt
Mu+Bt+Crd
Mu+Bt+Sil
Crd+Kfs+Grt+Opx
Crd+Kfs+Grt
Crd+Sil+Kfs+Grt
Sil+Kfs+Grt
Bt+Sil+Kfs
Mu+Bt+Ky
Bt+Sil+
Kfs+Grt
Bt+Crd+Kfs
Crd+Kfs+Opx
Bt+Crd+
Kfs+Grt
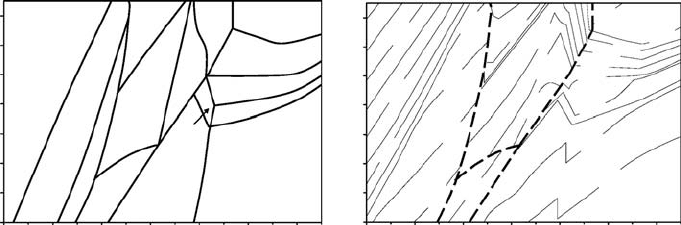
Fig. 2.2 Equilibrium mineral assemblages (a) and the corresponding density
(kg/m
3
) map (b) computed for a typical composition of metamorphosed aluminous
sediment (high-grade metapelite) on the basis of Gibbs free energy minimisation
(Gerya et al., 2001). Quartz, plagioclase and Fe-Ti oxides are present in all mineral
assemblages. Other minerals are: Bt = biotite, Chl = chlorite, Crd = cordierite,
Ep = epidote, Grt = garnet, Kfs = K-feldspar, Ky = kyanite, Mu = muscovite,
Opx =opthopyroxene, Sil =sillimanite. Heavy dashed lines in (b) indicate sharp
changes in density related to changes in the mineral assemblages.
where G
m(P, T)
is the molar Gibbs free energy (i.e. Gibbs potential) at a given P
and T; H
r
and S
r
are the enthalpy of formation and entropy respectively, of a
substance at standard pressure P
r
and temperature T
r
; C
Pr(T)
is the heat capacity as
a function of temperature at a standard pressure P
r
; V
(P, T)
is the molar volume of
substance as a function of pressure and temperature defined by a semi-empirical
EOS-function (such as, for example, Eq. 2.7).
Natural rocks typically contain several different minerals and therefore the den-
sity of a rock can be calculated from its mineralogical composition as follows
ρ
rock
=
n
i=1
ρ
i
X
i
, (2.12)
where n is the number of different minerals in the rock, X
i
is the volumetric
fraction of the i-th mineral in the rock and ρ
i
is the density of the i-th mineral as
a function of P and T. At any given P, T and chemical composition of the rock,
both the amount and the composition of the minerals can be computed using the
concept of thermodynamic equilibrium. According to this concept, the Gibbs free
energy of the rock in an equilibrium state corresponds to a global minimum. The
amount and composition of minerals can then be obtained from internally consistent
thermodynamic databases by using the so-called Gibbs free energy minimisation
approach (e.g. Karpov et al., 1976; Dorogokupets and Karpov, 1984, Connolly and
Kerrick, 1987; de Capitani and Brown, 1987). In this case, the density of rocks in
an equilibrium state (Sobolev and Babeyko, 1994;Petriniet al., 2001;Geryaet al.,

30 Density and gravity
2001;Kauset al., 2005) can be computed from Eqs. (2.8), (2.10), (2.11) and (2.12)
by using the densities and volumetric fractions of minerals composing a computed
equilibrium mineral assemblage (Fig. 2.2). Such an approach has recently been
used to constrain coupled petrological-thermomechanical numerical geodynamic
models (e.g. Gerya et al., 2004c, 2006; Tackley, 2008;Mishinet al., 2008)which
take into account changes of rock density and thermal properties during the course
of various geodynamic processes (we will discuss the details of this approach in
Chapter 17).
2.2 Gravity and gravitational potential
The density distribution in a continuous medium is inherently related to the grav-
itational field in this medium. This can be formulated in the form of a so-called
Poisson equation, which describes spatial changes in gravitational potential
inside a self-gravitating continuum
∂
2
∂x
2
+
∂
2
∂y
2
+
∂
2
∂z
2
= 4πGρ
(x,y,z)
, (2.13a)
or in a different symbolic notation
= 4πGρ
(x,y,z)
, (2.13b)
or
∇
2
= 4πGρ
(x,y,z)
, (2.13c)
where G = 6.672 × 10
−11
(N · m
2
)/kg
2
is the gravitational constant, ρ
(x,y,z)
is
the spatially variable density and (big delta), or ∇
2
is the Laplace operator
or Laplacian, which is a differential operator (like the divergence and gradient
operator from Chapter 1) often used in continuum mechanics for representing the
sum of second-order partial derivatives of a variable A
In 1D: A =∇
2
A =
∂
2
A
∂x
2
, (2.14a)
In 2D: A =∇
2
A =
∂
2
A
∂x
2
+
∂
2
A
∂y
2
, (2.14b)
In 3D: A =∇
2
A =
∂
2
A
∂x
2
+
∂
2
A
∂y
2
+
∂
2
A
∂z
2
. (2.14c)
The gravitational potential (J/kg), characterises the amount of potential energy
per unit mass for a given location, related to the interaction of the local mass with all
other surrounding masses. Another interpretation of is the amount of work needed
to be done to move a unit mass (i.e. overcoming its gravitational interactions with
surrounding masses) from a given location to infinity (where no interactions with
2.2 Gravity and gravitational potential 31
other masses occur). Since such work is always positive, the amount of potential
energy and therefore the gravitational potential is maximal at infinity. The relative
gravitational potential (i.e. changes in potential energy relative to infinity) will then
always be negative.
The Poisson equation (2.13) can be derived from Newton’s law of gravitation
which quantifies the gravitational attraction force f
g
acting between two bodies
with masses m
1
and m
2
, separated by a distance r
f
g
= G
m
1
m
2
r
2
, (2.15a)
or in 3-D vector notation
f
x1
= G
m
1
m
2
r
2
(
x
2
− x
1
)
r
, (2.15b)
f
y1
= G
m
1
m
2
r
2
(
y
2
− y
1
)
r
, (2.15c)
f
z1
= G
m
1
m
2
r
2
(
z
2
− z
1
)
r
, (2.15d)
g
x1
= G
m
2
r
2
(
x
2
− x
1
)
r
, (2.15e)
g
y1
= G
m
2
r
2
(
y
2
− y
1
)
r
, (2.15f)
g
z1
= G
m
2
r
2
(
z
2
− z
1
)
r
, (2.15g)
where f
x1
, f
y1
and f
z1
are components of the gravitational force vector
¯
f
1
acting
on mass m
1
and g
x1
, g
y1
and g
z1
are components of gravitational acceleration
vector
¯
g
1
felt by mass m
1
(in accordance with the second Newton’s law of motion,
acceleration can be defined as the amount of force per unit mass, g
i1
=
f
i1
m
1
).
The simplified explanation of such a derivation is the following. Firstly, we
should realize that local derivatives of gravitational potential by the spatial co-
ordinates are equal to the respective components of the gravitational acceleration
vector g, taken with a negative sign
∂
∂x
=−g
x
, (2.16a)
∂
∂y
=−g
y
, (2.16b)
∂
∂z
=−g
z
. (2.16c)
The minus sign in the right-hand side of equations (2.16a)–(2.16c) reflects the fact
that the potential energy should increase in the direction opposite to the direction
of the local gravity force, i.e. work contributing to the increase in potential energy
32 Density and gravity
has to be done by applying a force
f in the opposite direction compared to the
local gravity force
d
x
= f
x
dx =−g
x
dx, (2.17a)
d
y
= f
y
dy =−g
y
dy, (2.17b)
d
z
= f
z
dz =−g
z
dz, (2.17c)
where d
x
,d
y
and d
z
are increments in potential energy of a given unit mass
due to small changes (dx,dy and dz) in the respective coordinates of this unit
mass. According to Newton’s law of gravitation (Eq. 2.15), the acceleration (i.e.
gravitational force per unit mass)
¯
g = (g
x
,g
y
,g
z
) felt at any given point within a
continuum with coordinates x, y, z due to the gravitational attraction from surround-
ing masses is obtained by summing up (i.e. integrating) the accelerations exerted
by each small mass element (δm
i
), as follows
g
x
=
∞
i=1
G
δm
i
r
2
i
(
x
i
− x
)
r
i
, (2.18a)
g
y
=
∞
i=1
G
δm
i
r
2
i
(
y
i
− y
)
r
i
, (2.18b)
g
z
=
∞
i=1
G
δm
i
r
2
i
(
z
i
− z
)
r
i
, (2.18c)
r
i
=
(
x
i
− x
)
2
+
(
y
i
− y
)
2
+
(
z
i
− z
)
2
, (2.18d)
where x
i
, y
i
, z
i
are the coordinates of the i-th mass element and r
i
is the dis-
tance between this element and the given point. The divergence of the integrated
acceleration field at a given point can be then computed as follows (verify as an
exercise)
− =
∂
∂x
−
∂
∂x
+
∂
∂y
−
∂
∂y
+
∂
∂z
−
∂
∂z
= div(
¯
g) =
∂g
x
∂x
+
∂g
y
∂y
+
∂g
z
∂z
, (2.19a)
div(
¯
g) =
∞
i=1
Gδm
i
∂
∂x
x − x
i
r
3
i
+
∂
∂y
y − y
i
r
3
i
+
∂
∂z
z −z
i
r
3
i
, (2.19b)
div(
¯
g) =
∞
i=1
Gδm
i
3
(
x − x
i
)
2
r
5
i
−
1
r
3
i
+
3
(
y − y
i
)
2
r
5
i
−
1
r
3
i
+
3
(
z −z
i
)
2
r
5
i
−
1
r
3
i
, (2.19c)
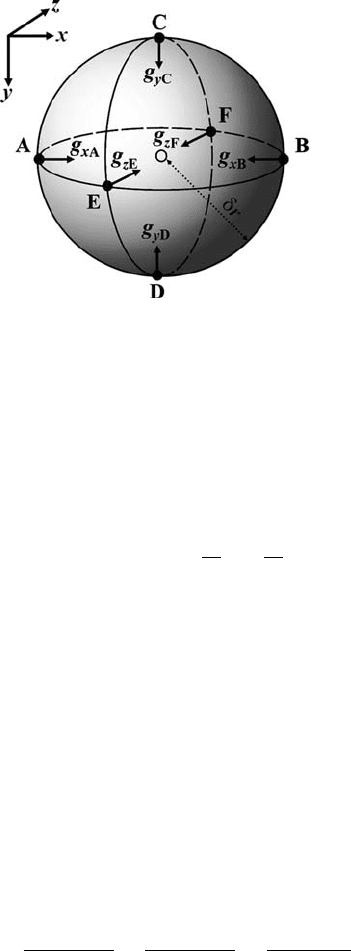
2.2 Gravity and gravitational potential 33
Fig. 2.3 Sphere of radius δr and mass δm considered for the derivation of the Pois-
son equation. Black arrows show respective components of gravitational accelera-
tion vector felt at the surface of the sphere in six different points (black dots, A, B,
C, D, E and F). Open circle shows the centre of the sphere in which the divergence
of the gravitational acceleration is computed.
and finally by using Eq. (2.18)
div(
¯
g) =
∞
i=1
Gδm
i
3
r
2
i
r
5
i
− 3
1
r
3
i
. (2.19d)
Note that differentiation is done with respect to the coordinates x, y, z of the given
point and not by those of the surrounding masses (x
i
, y
i
, z
i
) which are independent
of x, y, z.
For all r
i
= 0, Eq. (2.19d) is obviously zero. This means that the divergence of
gravitational acceleration at a given point is independent of the surrounding masses
and must come from the point itself (i.e. for x = x
i
, y = y
i
, z = z
i
and r
i
= 0).
Then one may restrict the volume of integration to a small sphere with mass δm
and radius δr centred on this point (Fig. 2.3). The divergence of acceleration for
this small sphere can be approximated by differences with the use of six points (A,
B, C, D, E and F, Fig. 2.3) located on the sphere as follows
div( g) =
g
xB
− g
xA
2δr
+
g
yD
− g
yC
2δr
+
g
zF
− g
zE
2δr
, (2.20)
where g
xA
−g
zF
are the respective components of the acceleration vector at different
points on the sphere’s surface. Density inside the sphere can be considered constant
since the mass inside the small sphere is uniform. Therefore, at the considered six
points, the respective components obviously coincide with the acceleration vectors
directed toward the centre of the sphere and can be computed from the common
34 Density and gravity
gravity formula for a sphere (see Turcotte and Schubert, 2002, Eqs. (5–1)–(5–15))
as
g
xA
= g
yC
= g
zE
= G
δm
δr
2
, (2.21a)
g
xB
= g
yD
= g
zF
=−G
δm
δr
2
. (2.21b)
Combining Equations (2.14)and(2.15) and taking into account that the average
density inside the sphere with volume V =
4
3
πδr
3
is given by ρ =
δm
V
=
3δm
4πδr
3
,
under the condition that δr tend to zero we obtain the Poisson equation (verify as
an exercise)
=−div(
¯
g) = 3G
δm
δr
3
= 4πGρ. (2.22)
It should be noted that our derivation is simplified and uses a standard expression
(Eq. (2.21)) for the gravitational acceleration at the surface of the sphere, which
can in turn be obtained by integrating Equation (2.18) for a point outside the sphere
(see Turcotte and Schubert, 2002, Eqs. (5–1)–(5–15) for details of this derivation).
It can also be shown on the basis of Gauss’s theorem that one can consider not only
a spherical geometry, but any arbitrary shape of the local mass and still obtain the
same Poisson equation.
Analytical exercise
Exercise 2.1
Molar Gibbs potential of periclase (MgO, molar mass m =0.0403044 kg/mol) is
given by the following equation (Gerya et al., 2004d)
G
m(P,T)
= H
r
+ V
r
+
3
i=1
c
i
[RT ln(
l
− e
i
) − H
i
e
oi
/(l − e
oi
)],
e
i
= exp[−(H
i
+ V
i
)/RT ],
e
oi
= exp(−H
i
/RT
r
),
= 5/4(
P
r
+ φ)
1/5
[(P + φ)
4/5
− (P
r
+ φ)
4/5
],
where R =8.314 J/mol is the gas constant, P
r
=100 000 Pa and T
r
=298.15 K
are the reference pressure and temperature respectively, and H
r
=−601 500.00
J, V
r
=1.122 28 ×10
−5
J/Pa, φ =30 179 500 000 Pa, c
1
=1.966 12, c
2
=4.12 756,
Programming exercises and homework 35
c
3
=0.536 90, H
1
=2966.88 J, H
2
=5621.69 J, H
3
=27 787.19 J, V
1
=
V
2
=3.52971 ×10
−8
J/Pa, V
3
=1.984 956 8 ×10
−6
J/Pa are empirical param-
eters.
Derive expressions for the density ρ, thermal expansion α and compressibility
β as functions of pressure and temperature (Fig. 2.1) using the equations given in
this chapter.
Programming exercises and homework
Exercise 2.2
Compute and visualise as colour maps with isolines (pcolor, contour, contourf)and
surfaces (surf, light, lighting) the density ρ, thermal expansion α and compress-
ibility β for periclase (Fig. 2.1) based on the equations derived for the analytical
exercise. Take a temperature interval from 100 to 4000 K and a pressure interval
from 10
9
to 10
11
Pa (1 to 100 GPa). Try also to define the Gibbs free energy
equation as an external function G
m(P, T)
and use differences instead of derivatives
to compute V
(P, T)
, ρ
(P, T)
, α
(P, T)
and β
(P, T)
.
ρ
(P,T)
=
m
V
(P,T)
, where
V
(P,T)
=
∂G
m(P,T)
∂P
T =const
≈
G
m(P,T)
P
=
G
m(P +P ,T )
− G
m(P,T)
P
α
(P,T)
=−
1
ρ
(P,T)
×
∂ρ
(P,T)
∂T
≈−
1
ρ
(P,T)
×
ρ
(P,T)
T
=−
1
ρ
(P,T)
×
ρ
(P,T+T )
− ρ
(P,T)
T
,
β
(P,T)
=
1
ρ
(P,T)
×
∂ρ
(P,T)
∂P
≈
1
ρ
(P,T)
×
ρ
(P,T)
P
=
1
ρ
(P,T)
×
ρ
(P +P ,T )
− ρ
(P,T)
P
,
where P and T are small increments in pressure and temperature, respectively.
Compare the results based on differences with your analytical solutions. An
example is in the code Periclase_EOS.m which calls function G_periclase.m.
Exercise 2.3
Load from files (fopen, fscanf, fclose) and visualise (pcolor) density maps (Mishin
et al., 2008) corresponding to the phase diagrams computed for pyrolite (mantle, file
m895_ro) and MORB (oceanic crust, file morn_ro) based on a Gibbs free energy
minimisation approach. Compute and visualise the density difference between
these two contrasting types of rocks at various P–T parameters. Check at which P
and T this difference is maximal/minimal.
The first nine positions in the data files are as follows
pl8951 350 350 800.003 10001.5 9.16904 4269.33 T(K) P(bar)
36 Density and gravity
where pl8951 denotes the rock identification (skip that); 350 and 350 are resolutions
for T and P respectively; 800.003 and 10001.5 are starting value for T (K) and P
(bar, 1 bar = 10
5
Pa), respectively; 9.16904 and 4269.33 are steps for T (K) and P
(bar), respectively; T (K) and P (bar) are P and T identifications (to skip). Further
data in the files are 350 ×350 maps of rock density (kg/m
3
)atvariableT (inner
cycle) and P (outer cycle). An example is in Density_map.m.
