Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

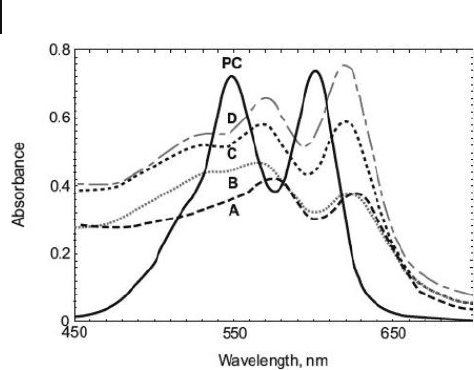
24.3 ). In aqueous solution, pinacyanol chloride ( PC ) showed absorption bands at
549 and 601 nm, but these were red - shifted to 564 and 610 nm, respectively, in
NCD micelles. Such a result suggested that NCDs could act as micellar hosts and,
potentially, also as water - soluble catalysts.
Chechik, Smith, and coworkers reported the synthesis of l - lysine based den-
dron - stabilized gold nanoparticles using the modifi ed one - phase Schiffrin reaction
( 5 ; Figure 24.2 ) [44] . The size of the nanoparticle core was found to decrease with
increasing dendron generation, a fi nding which contrasted with data reported for
other NCD systems [39, 41, 45] . These differences suggested that the structure and
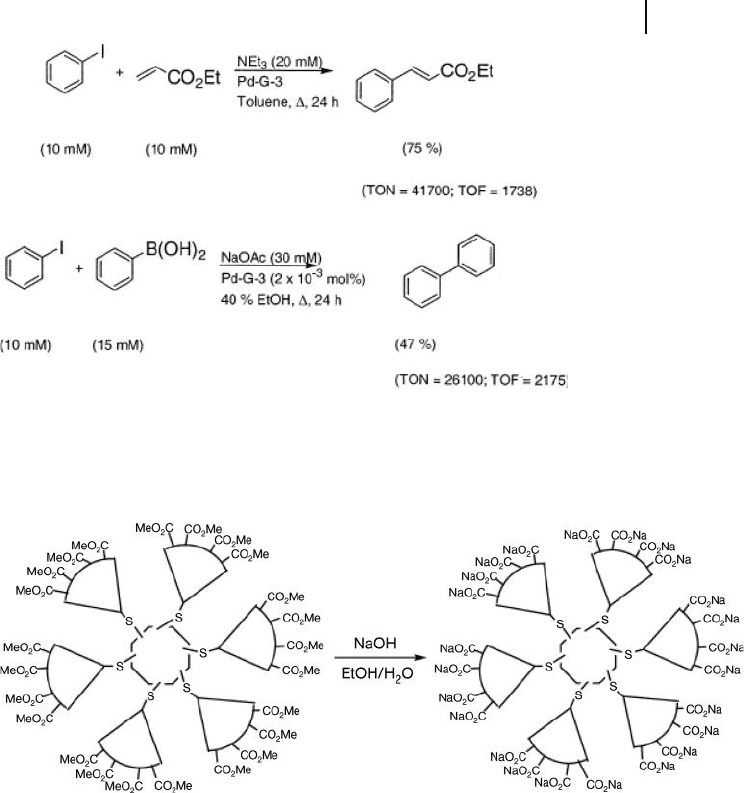
Scheme 24.3 Heck reaction (top) and Suzuki reaction
(bottom) with Pd - G - 3 NCDs. TON = turnover number;
TOF = turnover frequency. Reproduced with permission from
Ref. [42]; © 2003, The American Chemical Society.
Scheme 24.4 Basic hydrolysis of ester - terminated NCDs to
produce unimolecular micelles. Reproduced with permission
from Ref. [43]; © 2003, The American Chemical Society.
24.2 Synthesis of Nanoparticle-Cored Dendrimers via the Direct Method, and their Properties
749
750 24 Nanoparticle-Cored Dendrimers and Hyperbranched Polymers: Synthesis, Properties, and Applications
functionality of the dendron ligands must play an important role in determining
the characteristics of the gold core. Such a size relationship could be explained in
terms of steric effects, as the much more bulky higher - generation dendritic system
would be expected to pack more effi ciently around a small core, thus favoring a
smaller particle size. The thermal stability of the NCDs in solution was governed
by the extent of branching in the surrounding dendron ligands. A different order
of thermal ripening was observed at 120 ° C, which increased in size in the order
[G - 1] > [G - 2] > [G - 3].
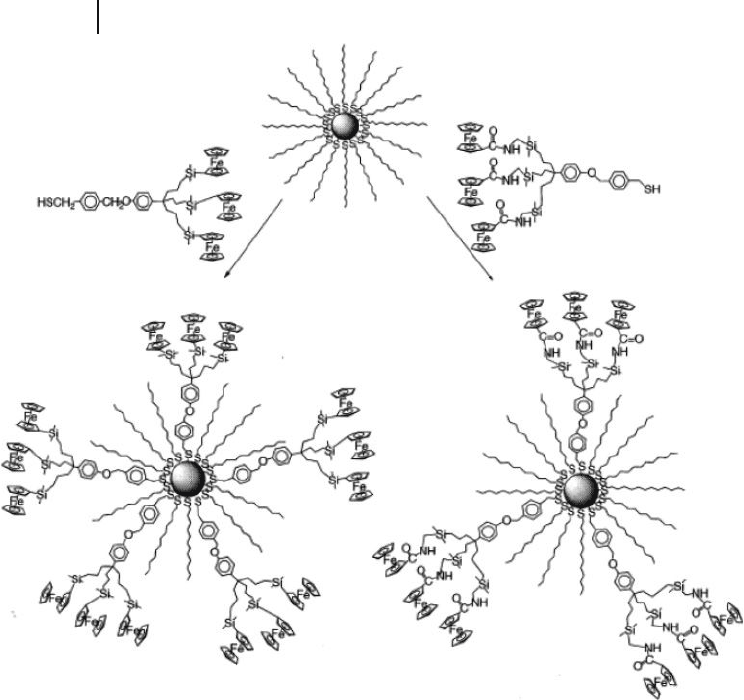
Astruc and colleagues synthesized redox - active NCDs using the Schiffrin reac-
tion from a 1:1 mixture of dodecanethiol and nonaferrocenyl thiol dendrons
(Scheme 24.5 ) [46, 47] . However, this approach resulted in NCDs without any open
catalytic sites on the nanoparticle core. Smaller dodecanethiols could be coassem-
bled on the nanoparticle surface, so that the integrity and solubility of NCDs was
suitable for their applications in the electrochemical sensing of anions. The gold
nanoparticle core was seen to be surrounded by ∼ 360 silylferrocenyl units, such
that the general structure of these NCDs closely resembled that of large metal-
lodendrimers. These redox - active NCDs were capable of selectively recognizing
HPO
24
−
anions and adenosine - 5 ′ - triphosphate (ATP
2 −
) with a positive dendritic
effect, even in the presence of other anions such as Cl
−
and HSO
4
−
. The titration
of NCDs using anions resulted in a shift of the cyclic voltammetry ( CV ) wave to
a less positive potential. The nonaferrocenyl thiol dendron - functionalized nano-
particles could be deposited on the Pt electrode by dipping it into the NCD solu-
tion. This modifi ed electrode was quite robust and was capable of recognizing
different anions. Moreover, the salt of these anions could be easily removed simply
Figure 24.3 Absorption spectra of pinacyanol chloride
(1 × 1 0
− 5
M) in water (PC) and in aqueous solutions of
(a) Au - G1(CO
2
Na), (b) Au - G2(CO
2
Na), (c) Au - G3(CO2Na),
and (d) Au - G4(CO2Na). [Au - G n (CO
2
Na)] were ∼ 0.1 mg ml
− 1
water. Reproduced with permission from Ref. [43]; © 2003,
The American Chemical Society.
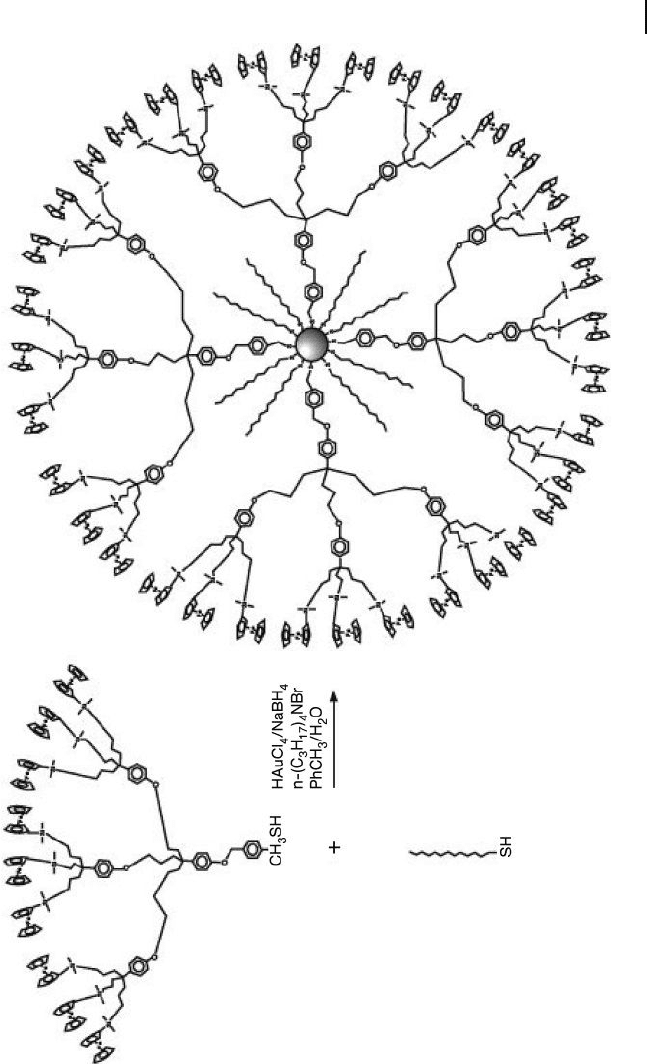
Scheme 24.5 Direct synthesis of NCDs containing the
nonaferrocenyl thiol dendron. Reproduced with permission
from Ref. [47]; © 2003, The American Chemical Society.
24.2 Synthesis of Nanoparticle-Cored Dendrimers via the Direct Method, and their Properties
751

752 24 Nanoparticle-Cored Dendrimers and Hyperbranched Polymers: Synthesis, Properties, and Applications
by dipping the electrode in CH
2
Cl
2
, the consequence being that the electrode could
be re - used many times.
Phenothiazine - terminated NCDs were prepared by Fujihara and coworkers
using the two - phase Schiffrin reaction ( 6 ; Figure 24.2 ) [48] . The gold nanoparticles
with a higher - generation dendron bearing a long chain alkanethiol at the focal
point had a smaller core size with a narrow size distribution. These NCDs under-
went the spontaneous formation of 1 - D arrays (Figure 24.4 ), and exhibited an
interesting one - electron transfer behavior. The intermolecular π – π stacking inter-
action of the thiol - terminated phenothiazine is believed to be the driving force for
this self - organization. The 1 - D assembly of redox - NCDs not only provides a good
model to study the size - dependent electronic and optical properties of metal nano-
particles, but may also play an important future role in nanoelectronics.
Dendrons with another focal group that is capable of metal complexation were
reported by Zheng et al. [45] . The Oct
4
N
+
- AuCl
4
in toluene was reduced by NaBH
4
in the presence of 4 - pyridone - functionalized dendrons ( 7 ; Figure 24.5 ) as a capping
reagent, and this resulted in stable, gold nanoparticle - cored dendrimers. There is
no known example of monolayer formations of pyridone derivatives on bulk gold
surfaces due to the chemical instability of such monolayers. Therefore, these
results suggests that the high surface curvature of the gold nanoparticles can
support the high - density packing of the capping ligands, which have only weak
metal surface - binding properties. An examination using TEM highlighted the
correlation between the particle size and the generation of dendrons, with higher -
generation dendrons producing larger particles ([G - 1], [G - 2], [G - 3] = 2.0, 3.3,
5.1 nm, respectively). However, the [G - 3] NCDs were less stable than [G - 1] and
[G - 2] NCDs, because the larger dendrons led to an increased open space between
the ligands. The resultant weaker force between the dendrons and the particle
caused a more rapid particle agglomeration of [G - 3] NCDs.
Figure 24.4 Transmission electron microscopy image of
one - dimensional arrays of phenothiazine - terminated NCDs.
Reproduced with permission from Ref. [48]; © 2006, The
Royal Society of Chemistry.
Dendrons with an ester focal point were also used for the synthesis of polyaryl
dendron - protected Pd nanoparticles ( 8 ; Figure 24.5 ) [49] . Briefl y, H
2
PdCl
4
was
phase - transferred into the organic phase using tetraoctylammonium bromide. The
dendrons were then added to the reaction mixture before addition of N
2
H
4
as
reducing agent. The resultant mixture was stirred under dry argon for an addi-
tional 24 h at room temperature. The Pd nanoparticle - cored dendrimers formed
had mostly a spherical shape, and an average core size which ranged from ∼ 10 to
∼ 70 nm. The Pd core size was seen to increases with the decreasing molar ratio
of dendrons to metal ions.
24.3
Synthesis of Nanoparticle - Cored Dendrimers by Ligand Exchange Reaction, and
their Properties and Applications
Nanoparticles with protecting monolayers composed of thiolate ligands can be
functionalized by a partial ligand - replacement [1] . In this exchange reaction, the
incoming ligands replace the thiolate ligands on nanoparticles by an associative
reaction, while the displaced thiolate becomes a thiol. Ligand - exchange reactions
of dodecanethiolate - protected gold nanoparticles with triferrocenyl thiol dendrons
in dichloromethane were fi rst reported by Astruc et al. (Scheme 24.6 ) [46, 47] .
Although an excess of functional thiol dendrons was used, the percentages of
dendron thiols introduced as ligands in dodecanethiolate - protected gold nanopar-
ticles were less than 5% of the overall ligands. The limit of the ligand - exchange
reaction was even greater with larger dendrons. Attempts to synthesize NCDs with
nonaferrocenyl thiol dendrons resulted in the incorporation of very small amounts
of dendrons (less than one per nanoparticle). As the rate of ligand - exchange
Figure 24.5 Structure of dendrons with other functional
groups used for the synthesis of nanoparticle - cored
dendrimers.
24.3 Synthesis of Nanoparticle-Cored Dendrimers by Ligand Exchange Reaction, and their Properties 753
754 24 Nanoparticle-Cored Dendrimers and Hyperbranched Polymers: Synthesis, Properties, and Applications
between thiols is dependent on the chain length and/or steric bulk of the initial
monolayers on nanoparticles, the bulky or very small incoming ligands are diffi cult
to replace the original ligands on the nanoparticle surface due to either kinetic
(e.g., steric hindrance) or thermodynamic effects, respectively. Thus, the main
short - coming of the ligand - exchange reaction, when using larger dendrons, was
in fact quite consistent with previous results on ligand - place exchange reactions
of alkanethiolate - protected nanoparticles [1, 3] . In contrast, the exchange between
ligands having different functional groups was much more effi cient.
Peng et al. synthesized hydrophilic NCDs by the ligand - exchange of citrate -
capped gold nanoparticles using hydroxy - functionalized dendron thiols ( 9 ; Figure
24.6 ) [50] . The citrate reduction of HAuCl
4
in water led to citrate - capped gold
nanoparticles with core sizes that ranged from 10 to 150 nm [3] . Such nanoparticles
Scheme 24.6 Synthesis of NCDs using the thiol - ligand
exchange reaction. Reproduced with permission from Ref.
[47]; © 2003, The American Chemical Society.
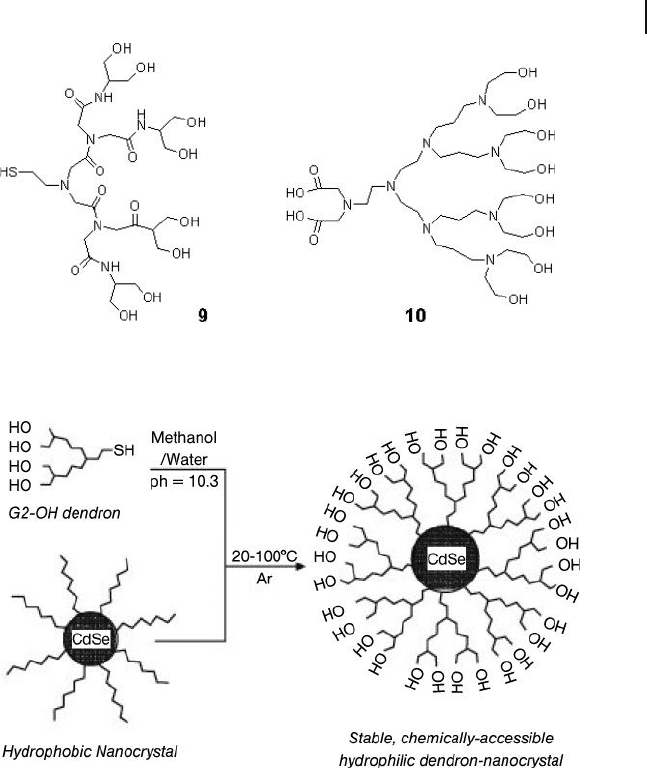
are typically useful when a rather loose shell of ligands is required around the gold
core for ligand - exchange [64] . Peng also performed ligand - exchange reactions of
trioctylphosphine oxide ( TOPO ) - capped CdSe nanoparticles with hydroxy - func-
tionalized dendron thiols (Scheme 24.7 ) [50, 51] . Dendron thiols can rather easily
replace the loosely bound TOPO groups, which have a weak binding property with
CdSe nanoparticles. The chemistry related to CdSe NCDs can be applied for devel-
oping photoluminescence - based labeling reagents for biomedical applications.
The photochemical, thermal, and chemical stability of both CdSe and Au NCDs
was exceptionally good compared to that of the corresponding alkanethiolate -
Figure 24.6 Structure of hydroxy - functionalized dendrons
used for the synthesis of nanoparticle - cored dendrimers.
Scheme 24.7 Schematic process for converting hydrophobic
semiconductor nanoparticles into hydrophilic and chemically
processable NCDs. Reproduced with permission from Ref.
[50]; © 2002, The American Chemical Society.
24.3 Synthesis of Nanoparticle-Cored Dendrimers by Ligand Exchange Reaction, and their Properties
755
756 24 Nanoparticle-Cored Dendrimers and Hyperbranched Polymers: Synthesis, Properties, and Applications
protected nanoparticles. The stability of CdSe NCDs could be further enhanced by
a global crosslinking of the dendron ligands around a nanoparticle core [51] . Such
crosslinking of alkene - terminated CdSe NCDs was achieved via ring - closing
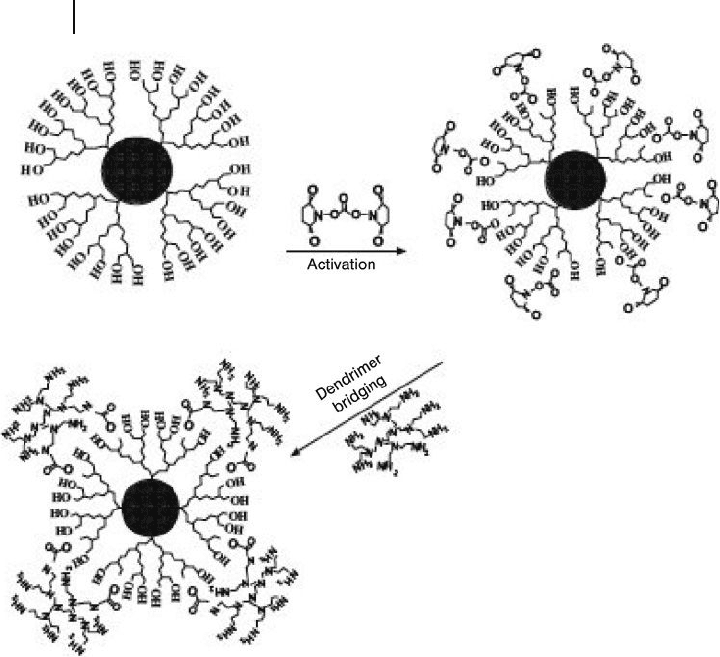
metathesis, and led to each nanoparticle being sealed in a dendron box. Another
strategy – dendrimer bridging – was also reported by Peng for the simultaneous for-
mation and functionalization of a biocompatible and bioaccessible dendron box
with a semiconductor nanoparticle core [52] (Scheme 24.8 ). The dendrimer bridg-
ing involved an activation of the hydroxyl terminal groups of CdSe NCDs with a
homobifunctional crosslinker, N,N - disuccinimidyl carbonate, followed by a
crosslinking reaction with [G - 2] polyamidoamine ( PAMAM ) dendrimers.
The resulting amine box nanoparticles were very stable chemically, thermally,
and photochemically. In addition, the amine groups on the surface of the box
nanoparticles provided reactive sites for the conjugation of biological entities.
Scheme 24.8 Synthesis of amine - functionalized box
nanoparticles. Reproduced with permission from Ref. [51];
© 2003, The American Chemical Society.
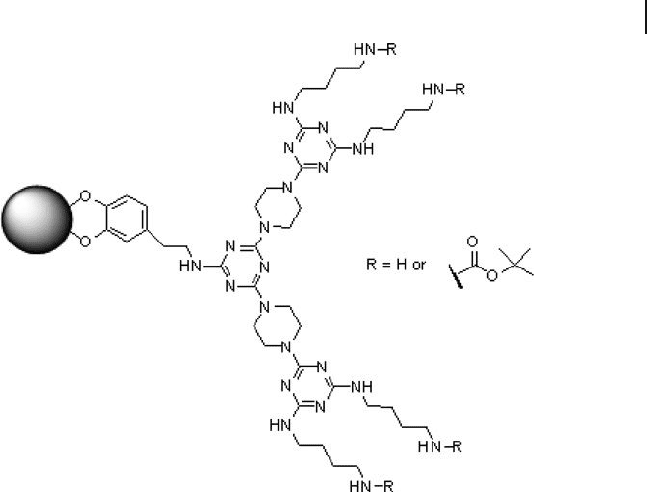
Figure 24.7 Simanek (melamine) - type dendron - coated iron oxide nanoparticles.
Bioconjugation and biodetection using box nanoparticles were each successfully
demonstrated with the avidin – biotin system [52] .
Liu and Peng reported the synthesis of highly luminescent water - soluble CdSe/
CdS core – shell NCDs by ligand - exchange reactions [53] . A dendron ligand with
eight hydroxyl terminal groups and two carboxylate anchoring groups was found
to replace alkylamine ligands on CdSe/CdS nanoparticles in toluene, and to
convert the particles to water - soluble NCDs ( 10 ; Figure 24.6 ). The resultant water -
soluble NCDs retained 60% of the photoluminescence value of the alkylamine -
protected CdSe/CdS core – shell nanoparticles in toluene. Compared to the dendron
thiol - protected NCDs, the photoluminescence value of these NCDs was much
higher (about sixfold). Moreover, the UV - brightened photoluminescence could be
retained for several months.
Dendron (with a shell of Simanek) - fuctionalized superparamagnetic (iron oxide)
nanoparticles were prepared by Gao et al. using ligand - exchange methods (Figure
24.7 ) [54] . The resulting Fe
2
O
3
NCDs exhibited a switchable solubility in a variety
of solvents, depending on the terminal groups of the dendritic shells, and were
examined as soluble matrices for supporting magnetically recoverable homogene-
ous Pd catalysts for Suzuki crosscoupling reactions in organic solvents. For this,
Gao immobilized a Pd - triphenyl phosphine moiety to the termini of dendron -
protected iron oxide nanoparticles for catalysis. The Fe
2
O
3
NCDs were also inves-
tigated as potential contrast agents for magnetic resonance imaging ( MRI ) in
aqueous media.
24.3 Synthesis of Nanoparticle-Cored Dendrimers by Ligand Exchange Reaction, and their Properties 757

758 24 Nanoparticle-Cored Dendrimers and Hyperbranched Polymers: Synthesis, Properties, and Applications
24.4
Synthesis of Nanoparticle - Cored Dendrimers by Dendritic Functionalization, and
their Properties and Applications
The direct synthesis of NCDs was somewhat problematic because it required a
large excess of dendronized thiols or disulfi des, especially for the synthesis of
NCDs with a small and monodispersed nanoparticle core [39 – 41] . The direct
method also provided little control over the nanoparticle core dimension. NCDs
with different core size were produced, when dendrons with different sizes (or
generations) were used. In addition, the NCDs synthesized by the direct method
often contained small amounts of trapped tetraoctylammonium bromide that
could not be removed completely, even by repeated extraction with solvent. The
indirect method using the ligand - exchange reaction was also somewhat limited
considering a low exchange rate, especially for the synthesis of metal nanoparticle -
cored dendrimers, as described in Section 24.3 . A more convenient and cost - effi -
cient synthetic methodology for the synthesis of NCDs with controlled particle
core sizes, generations, and dendritic wedge density has been considered highly
desirable for the basic understanding of structure – property relationships of these
nanostructures.
The present authors ’ approach is based on a strategy in which the synthesis of
monolayer - protected nanoparticles is followed by building the dendrimer architec-
ture on the nanoparticle surface, using either the convergent or divergent approach
(Scheme 24.9 ). A convergent synthesis of NCDs ( 11 ) can be accomplished by the
coupling of reactive nanoparticles with functionalized dendrons. As the reaction
only takes place at the functional groups on the surface of nanoparticles, this
approach eliminates the need for a large excess of functionalized dendrons, and
also maintains an intact core size for the synthesis of NCDs with different interior
layers (generations) and dendritic wedge densities. The divergent approach
Scheme 24.9 General reaction schemes for the synthesis of
nanoparticle - cored dendrimers by ( 11 ) convergent and ( 12 )
divergent approaches.
