Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

The phases of austenite, pearlite, and ferrite are relatively soft; hence, the
observed high hardness of steels is obtained through processing of these materials.
For instance, hypoeutectoid steel may be heated to form austenite and then slowly
cooled so the cementite/ferrite phases may be worked into desired shapes. If the
material is re-austenized and quickly quenched to room temperature, a very hard
phase known as martensite is formed. Some of the remaining pearlite and ferrite
phases (if present) would still remain in the matrix. Hence, only when the steel has
been heated to temperatures sufficiently high to convert all of the ferrite into
austenite, that quenching will result in pure martensitic steel. It should be noted
that the martensite phase does not appear in the above Fe–C phase diagram since it is
a non-equilibrium phase.
The temperature range is not the only crucial variable affecting the properties of
the material, but rather the rate of heating and cooling. For example, when austenitic
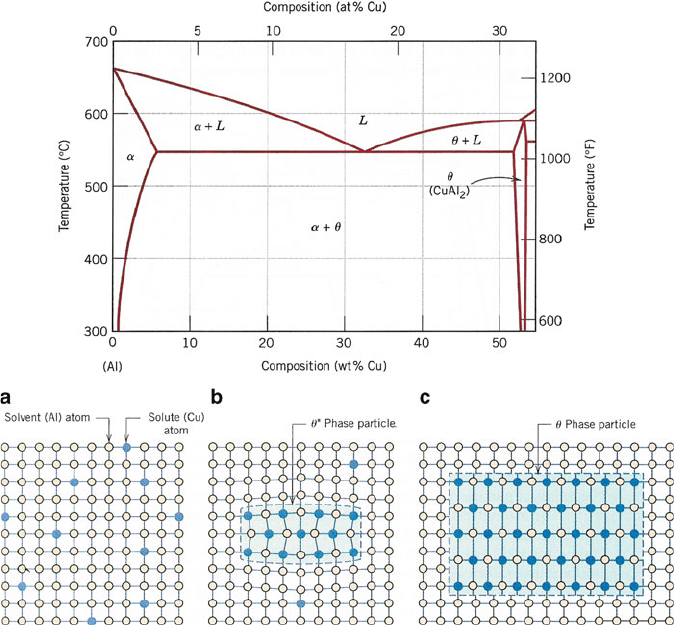
Figure 3.21. Top: The Al-rich side of the Al-Cu phase diagram. Bottom: schematic of the formation of
the equilibrium precipitate s phase: (a) a supersaturated a solid solution, (b) a transition, y
00
, precipitate
phase, and (c) the precipitate y phase within the a-matrix phase. Reproduced with permission from
Callister, W. D. Materials Science and Engineering: An Introduction, 7th ed., Wiley: New York, 2007.
Copyright 2007 John Wiley & Sons, Inc.
188 3 Metals
steel is very slowly cooled to room temperature, the resulting solid will be soft and
malleable. However, when the same steel is rapidly quenched in cold water to
temperatures less than 250
C, the normal phase transformations to pearlite/ferrite
or pearlite/cementite (depending on %C present) are suppressed. Rather, the g-Fe
phase is conver ted to martensite, the hardest and strongest of all possible Fe–C
microstructures. Intere stingly, the formation of martensite may also occur through
fast quenching of other austenitic mixtures, such as ferrite- or cementite-rich
austenite (Eq. 16):
g Fe þa Fe
fg
!
rapid cooling
martensite þ a Fe
g Fe þ Fe
3
C
fg
!
rapid cooling
martensite þ Fe
3
C
ð16Þ
To summarize, the relative hardness of the various phases discussed thus far
(Brinell hardness values in parentheses): martensi te (300–700) > tempered mar-
tensite (300–450) > bainite (ca. 400) > fine pearlite (100–300) > coarse pearlite
(100–220) > spheroidite (90–180). The hardness and brittleness of cementite is
much greater than ferrite, whereas the latter has significantly greater ductility.
In order to predict the resultant phase arising from varying the cooling rate of
austenitic steel, one would use a time–temperature-transformation (TTT) curve
(e.g., Figure 3.22). To generate a TTT diagram, thin metal specimens are suitably
heated to form austenitic steel. This temperature is held at varying temperatures to
ensure full conversion of the microstructure to austenite. This is to allow the metal
carbides to fully dissolve in austenite; incomplete conversion of carbides will result
in ferrite grains that will ultimately weaken the material. The austenized specimens
are then removed at specific times and quenched in cold water. Using optical and
electron microscopies, the microstructure of the products is determined (i.e., pure
martensite, ferrite, pearlite, etc.). As one would expect, a large number of samples
are required to determine the time intervals required for the initial and full transfor-
mation of austenite to other phases, making this process an extremely labor-inten-
sive exercise.
As shown in Figure 3.22a, cooling austenitic steel at a rate fast enough to avoid
the nose of the transformation curve (i), will result in 100% martensite. Cooling
curve (ii) is tangent to the nose of the TTT plot. This will also result in 100%
martensite, but with significantly lower internal stresses and distortions than (i).
Such a tangent line represents the slowest cooling rate that prevents formation of the
non-martensitic decomposition products of pearlite or bainite, referred to as the
critical cooling rate (CCR). A tangent to the 50% transformation curve (cooling
curve (iii)) will result in a mixture of 50% pearlite and 50% martensite. Cooling curve
(iv) represents relatively slow cooling to ca. 600
C, where 50% of the austenitic steel
is converted to pearlite (i.e., still above the pearlite/bainite threshold temperature
of 538
C). Subsequent fast cooling to ca. 450
C, and isothermal equilibration for
a few hundred seconds converts the remaining austenite into bainite. Hence, the
final product from (iv) will be 50% pearlite and 50% bainite. The cool ing curve
(v) will result in 100% bainite, since fast cooling to ca. 500
C avoids the nose of
the TTT diagram and is held isothermally below the pearlite/bainite boundary.
3.2. Metallic Structures and Properties 189
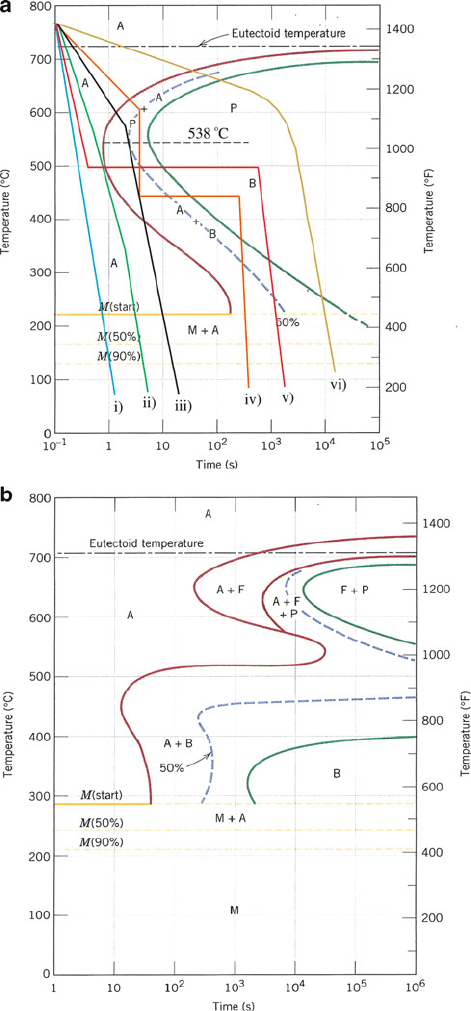
Figure 3.22. Time-temperature-transformation (TTT) diagrams for (a) austenitic steel, and (b) an alloy
steel (type 4340); A ¼ austenite, B ¼ bainite, P ¼ pearlite, M ¼ martensite, F ¼ proeutectoid ferrite.
Reproduced with permission from Callister, W. D. Materials Science and Engineering: An Introduction,
7th ed., Wiley: New York, 2007. Copyright 2007 John Wiley & Sons, Inc.
190 3 Metals
Finally, cooling curve (vi) illustrates very slow cooling (e.g., furnace cooling), which
will result in 100% pearlite.
Solute hardening
The introduction of foreign species into the metallic lattice through alloy formation
introduces alien crystallites that will also impede slip in steel crystals by increasing
the lattice energy in the vicinity of the dopant. Strengthening occurs since more
work is required to propagate a dislocation through these areas. In particular, if the
alloying agent is carbon, hard crystallites of iron carbide may form that changes the
microstructure. By comparison, austenite usually does not contain iron carbide, and
is quite susceptible to slip.
From an analysis of various types of steels, only the following carbides will be
present: Fe
3
C, Mn
3
C, Cr
23
C
6
,Cr
7
C
3
,Fe
3
Mo
3
C, Fe
3
W
3
C, Mo
2
C, W
2
C, WC, VC,
TiC, NbC, TaC, Ta
2
C, and ZrC. The occurrence of these species will depend on the
type and concentration of the transition metal dopants within the iron lattice. For
interstitial carbides, the size of the metal atoms will govern the type of carbide
formed. In gener al, the metal radius must be >1.35 A
˚
(e.g., Ti, Zr, Hf, V, Nb, Ta,
Mo, and W) to generate an interstitial vacancy large enough to accommodate C
atoms. Metals with smaller radii (e.g., Cr, Mn, Fe, Co, Ni) do not form MC species,
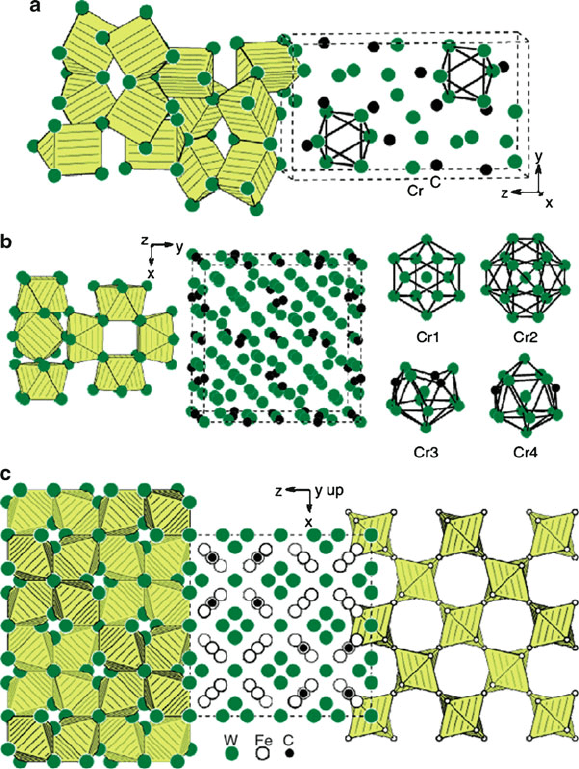
and form carbides with relatively com plex crystal structures (Figure 3.23 ). It should
be noted that metal carbides do not generally exist as isolated pure species. That is,
carbides of all alloying elements will exist as clusters that also contain iron. Further,
when several carbide-forming dopants are present that share the same crystal
structure, the resultant carbide will be present as a combination of those elements.
As an example, steel containing Cr and Mn dopants will contain particulates of the
complex carbide (Cr, Mn, Fe)
23
C
6
, rather than isolated Cr
23
C
6
and Mn
3
C species.
We have seen that only certain transition metals will form stable carbides; as a
relevant digression, let us consider the chemical rationale behind such reactivity.
The general trend for increasing carbide-forming ability of transition metals is:
Fe < Mn < Cr < Mo < W < V < Nb < Ta < Ti < Zr < Hf
If one follows this sequence using the Periodic Table, this grouping consists of
early transition metals that are relatively electron deficient. As you may recall, the
valence shell of zero-valent transition metals in a crystal lattice is [(ns
2
)((n 1)d
x
)].
In the bulk solid state, the outer s electrons are completely delocalized, whereas the
wave functions of the d electrons remain localized on the respective metal atoms.
When a carbon atom enters the crystal field it behaves as a ligand toward the metal,
with the ligand and metal electrons electrostatically interacting causing the d orbitals
to lose their original degeneracy.
[10]
Since this is an electrostatic effect, stronger
metal–carbon bonds will result from more diffuse metal d orbitals (5d vs.4dvs. 3d),
and metals with fewer d electrons (i.e., both corresponding to less electron–electron
repulsions between ligands and the metal ).
The transference of electron density from the metal to carbon will result in the
formation of a strongly polar covalent bond, between carbide ions (C
x
n
) and
3.2. Metallic Structures and Properties 191
transition metal ions. It should be noted that only the portions of the alloying
elements and carbon that cannot be dissolved in austenite at a given temperature
may be used in carbide formation. Two types of carbides are possible. If the atomic
radius ratio of carbon/metal is <0.59, an interstitial phase will result; otherwise,
carbides of complex compositions will form, having a different crystal lattice from
Figure 3.23. Crystal structures of typical carbides in steels. Shown are (a) orthorhombic Cr
7
C
3
(space
group: Pnma) (b) cubic Cr
23
C
6
(space group: Fm3m), (c) cubic Fe
3
W
3
C (space group: Fd3m), (d)
orthorhombic Fe
3
C (space group: Pnma), and (e) cubic TiC (space group: NaCl) and hexagonal W
2
C
(space group: CdI
2
). Reproduced with permission from Handbook of Ceramic Hard Materials, Riedel, R.
ed., Vol. 1. Copyright 2000 Wiley-VCH. (http://www.hardmaterials.de/html/_crystal_structures.html).
192 3 Metals
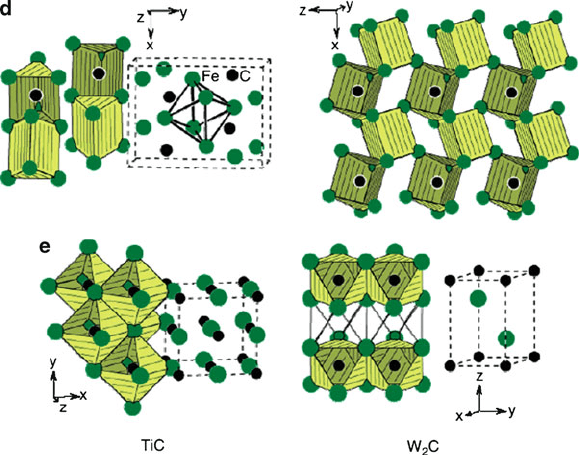
the host metal. The latter carbides are characteristic of Cr, Mn, and Fe, which have
five or more d electrons and a correspondingly weaker interaction with ligands such
as carbon. Hence, such complex carbides (e.g., Figure 3.23a–d) will have lower
melting points and hardness than analogous interstitial carbides of the 5d and/or
early transition metals (e.g.,W
2
C used as cutting blades).
It is interesting to note that alloying metals that are present in steel will cause stark
changes in TTT diagrams (Figure 3.22b). In particular, the nose of the austenite/
pearlite transformation will be compres sed, allowing for pearlite (and martensite)
formation at slower cooling rates. In addition, a second bainitic nose will appear in
the TTT diagram. For a given steel with constant %C, as the concentration of
metallic dopant s increase, there will be a decrease in the temperature required for
the onset of martensite formation, M
s
(Eq. 17):
M
s
ð
CÞ¼539 423ð%CÞ30:4ð%MnÞ17:7ð%NiÞ
12:1ð%CrÞ7:5ð%MoÞ
ð17Þ
The greatest effects are seen for the austenite-forming elements of C, Mn, and Ni
where even small concentrations result in a sharp decrease in M
s
. Whereas pure g-iron
may be converted to martensite at temperatures in excess of 500
C, hypereutectoid
steel is not transformed to martensite until a temperature of ca.160
C is reached during
quenching. For steels with carbon concentrations above 0.7% and/or high dopant
Figure 3.23. Continued
3.2. Metallic Structures and Properties 193

concentrations, martensite may be formed at temperatures well below 0
C. Hence,
high-C steels must be quenched in low-temperature media (e.g., dry ice/acetone, liquid
nitrogen) to ensure full conversion of austenite to martensite.
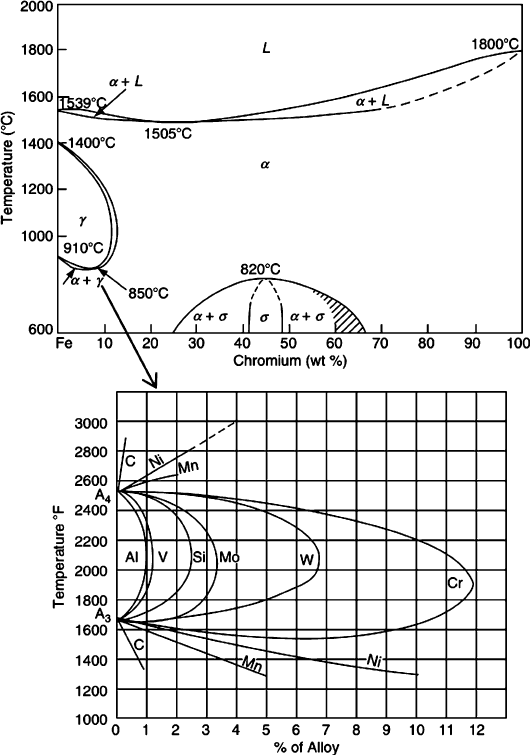
The effect of alloying elements may be understood by examining the austenite/
ferrite regions of the Fe–C phase diagram with respect to the alloy concentrations
(Figure 3.24). Ferrite-forming elements such as Al, Si, W, Cr, and Mo result in a
contraction of the austenite region, forming a gamma loop. By contrast, austenite-
stabilizing elements such as C, N, Mn, Ni, and Cu cause an expansion of the
austenitic phase boundary. Austenite stabilizers inhibit the nucleation and growth
of ferrite and pearlite/bainite phases, assisting in the formation of pure martensite
upon quenching. In general, since bcc ferrite contains more voidspace than fcc
austenite, larger interstitial dopant s may be incorpor ated into these lattices. Hence,
ferrite stabilizers tend to be larger in contrast to the smaller size of austenite
stabilizers. Most importantly, in accord with the “like dissolves like” principle,
bcc and fcc dopants will tend to stabilize ferrite and austenite, respectively.
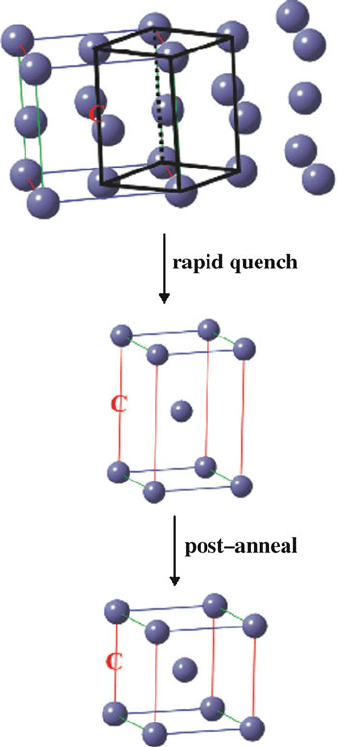
A slow cooling rate will give the greatest opportunity for controlled atomic
migration within the lattice, and growth of large ordered crystallites. However, in
the rapid non-equilibrium conditions used to form martensite, there is no time for
carbon diffusion to occur; this yields a supersaturated solution, with >2 wt.% C
present as an interstitial impurity. During this phase transition, the fcc lattice of
austenite is transformed to a distorted bcc lattice, commonly referred to as body-
centered tetragonal bct (Figure 3.25). The degree of distortion from a perfect bcc
lattice (cubic lattice axes ratio, c/a ¼ 1) is amplified with increasing carbon con-
centration (Eq. 18):
c
a
¼ 1 þ 0.045(wt% C)ð18Þ
The fcc–bct conversion, known as the Bain transformation, is a diffusionless
process. That is, unlike the previous high-temperature conversions we saw earlier
(e.g., austenite to ferrite), martensite can form at temperatures significantly below
room temperature, within 1 10
7
s. Such a fast growth rate precludes the decipher-
ing of the exact mechanism for the nucleation and growth of martensite. However,
leading theories suggest that the growth initiates from dislocations in the solid.
[11]
Tempering
Even though martensite exhibits a high hardness, the as-quenched material is much too
brittle and highly stressed for structural applications. The ductility and toughness of
martensite is greatly improved through post-annealing, a process known as tempering.
This process relieves stresses in the solid through conversion of bct martensite into bcc
ferrite, with precipitation of iron carbide particulates. It is important to note that the
annealed structure is not simply pearlite/ferrite, but is best referred to as tempered
martensite. During the annealing process for martensite, a number of key transforma-
tions occur:
(i) 50–250
C: Interstitial carbon atoms in martensite begin to diffuse within the
bct lattice. This results in precipitation hardening from the formation of
194 3 Metals
hexagonal e-iron carbide crystallites, Fe
x
C(2< x < 3). The martensite bct
crystal lattice begins to lose its tetragonality.
(ii) 250–350
C: Decomposition of retained austenite to fine aggregates of ferrite
plates (acicular ferrite) and cementite crystallites. This intermediate micro-
structure is referred to as bainite, nucleating from the surface of ferrite crys tal-
lites. As the temperature approaches 300
C, cementite begins to dominate the
Figure 3.24. The phase diagram for the Fe–Cr system, illustrating the expanded ferrite region, relative to
the normal Fe–C diagram. Below is shown the “gamma loop” illustrating the size of the austenite region
from the presence of various dopants. Reproduced with permission from (top) Steels: Microstructure and
Properties, Honeycombe, R. W. K.; Bhadeshia, H. K. D. H.; 2nd ed.; Copyright 1995 Elsevier, and
(bottom) Basic Metallurgy: Volume I, Principles, Grosvenor, A. W.; 3rd ed.; American Society for
Metals: Cleveland, OH, 1958. All rights reserved (http://www.asminternational.org).
3.2. Metallic Structures and Properties 195
microstructure at the expense of the e-Fe
x
C particles. At temperatures
approaching 350
C, the bct lattice is transformed to ferritic bcc.
(iii) 350–700
C: The cementite particles undergo a coarsening process (between
300
C and 400
C), and spheroidization (near 700
C). These processes drasti-
cally reduce the hardness of the material, but improve overall ductility and
brittleness characteristics (more desirable for particular appl ications).
Hence, to obtain high-strength tempered steels, it is essential to anneal at low
temperatures (<350
C). As one might expect, the presence of alloying elements
Figure 3.25. Crystallographic representation of the phase transformation from austenite to martensite.
Two neighboring fcc unit cells of austenite associate, resulting in a body-centered tetragonal (bct) unit
cell. A postanneal known as tempering converts the bct structure to a-Fe. Also shown is the placement of
an interstitial carbon atom, remaining in an octahedral site among lattice iron atoms.
196 3 Metals
will have a dramatic effect on the microstructure during annealing. For instance, if
1–2 wt.% Si is present, e-iron carbide particles are stabilized up to temperatures of
400
C, yielding a much harder material at elevated temperatures. Further, transition
metals such as Cr, Mo, V, W, and Ti will form stable carbides with higher enthalpies
of formation than Fe
3
C, typically at temperatures between 500
C and 600
C. A high
temperature is required due to the relatively low diffusivity of the alloying elements
that must substitutionally diffuse through the iron lattice. By contrast, int erstitial
dopants such as C, N, and B move between the iron lattice sites with a much greater
diffusivity. It is important to note that the alloy carbides remain as fine suspensions
even after prolonged tempering. This results in substantial strengthening referred to
as secondary hardening.
One serious drawback of the above austenization/rapid quench method for mar-
tensite formation is the possibility of distor ting and crackin g the metal due to the
rapid cooling event. During the quenching process, thermal stresses arise from the
varying cooling rates experienced by outer and interior areas of the steel. In addition,
there is a volume change when austenite is transformed to martensite. Two methods
that have been used to reduce quenching stresses are martempering and austemper-
ing (Figure 3.26). Martempering allow s the transformation of austenite to martensite
to take place at the same time throughout the structure of the metal part. By using
interrupted quench, the cooling is stopped at a point above the martensite transf or-
mation region to allow sufficient time for the center to cool to the same temperature
as the surface. Then cooling is continued through the martensite region, followed by
the usual tempering process. By comparison, in austempering, the austenized steel is
quenched at a rate faster than that required for pearlite formation, but above the
temperature required for martensite growth. Hence, rather than transforming to
martensite, the center and surface are converted to bainite – a strong material that
shares the hardness of martensite with the toughness of pearlite.
Surface hardening
The above changes in the microstructure upon annealing do not only apply to the
bulk material, but also for the surface. If an iron material is placed at high tempera-
ture in the presence of carbon vapor, a procedure known as carburization occurs,
where carbon atoms diffuse into the surface of the steel, increasing the surface
hardness. There must be careful control of the annealing atmosphere; if the steel is
brought into cont act with an oxidizing atmosphere, decarburization of the surface
will occur through preferential formation of CO
2
.
Other surface hardening techniques introduce nitrogen to steels containing metals
such as Al, Cr, and V that form stable nitrides (Eq. 19). Surface hardening techni-
ques add a variety of attractive properties to steel components such as increas ing
wear and stress resistances, decreasing the odds of fracturing, and increasing
corrosion resistance.
4Cr þ 3NH
3ðgÞ
!
600
C
Cr
4
N
3
þ9=2H
2ðgÞ
ð19Þ
Strengthening of the exterior of a material may be achieved through either
diffusional incorporation of dopants (e.g., B, C, N), or annealing selective portions
3.2. Metallic Structures and Properties 197
