Elder K. Human preimplantation embryo selection
Подождите немного. Документ загружается.

HUMAN PREIMPLANTATION EMBRYO SELECTION
a link between blastomere size and multinucleation.
This is supported by the findings of Hnida et al.
18,24
One possible explanation for the impaired qual-
ity of these embryos could be that the presence of
unequally sized blastomeres indicates that they have
divided in an asynchronous or asymmetrical pattern,
or that one or more of the cells have ceased to divide
(Figure 8.3). However, the fact remains that we are
currently at the stage of having only limited knowl-
edge about the order of magnitude of difference
between blastomere size that is required in order to
compromise the embryo’s developmental potential.
MULTINUCLEATION
Transfer of embryos with multinucleated blastomeres
has been shown to be associated with decreased
implantation, pregnancy, and birth rates.
25–27
Further-
more, multinucleated embryos have increased rates of
chromosomal abnormalities.
28–30
Thus, multinucle-
ated embryos should be excluded from transfer
28,29
and assessment of nuclear status should be included
in embryo scoring systems.
25,26
Van Royen et al
27
found multinucleation in 34% of a cohort of embryos
from patients undergoing IVF or ICSI treatment.
Hnida et al
18
showed that the volume of multinucle-
ated blastomeres was significantly larger than their
mononucleated sibling blastomeres (22% in 2-cell
and 30% in 4-cell embryos). These findings support
other studies indicating that an intraembryonic vari-
ation in blastomere diameters of more than approx-
imately 25% is associated with increased rates of
multinucleation and chromosomal abnormalities.
19,30
However, these studies did not measure the precise
blastomere sizes. It has previously been suggested
that multinucleated blastomeres can originate from
an uncoupling of processes that control karyokinesis
and cytokinesis, resulting in duplication of the nucleus
without subsequent cell cleavage.
17,31
The conse-
quence of this would be a multinucleated blastomere
without the size reduction from cell cleavage, thus
retaining the size of the previous cell generation.
These speculations are supported by the findings of
Hnida et al
18,24
demonstrating that a multinucleated
4-cell blastomere was approximately the same size as
a non-multinucleated 2-cell blastomere (Figure 8.6).
of fragmentation based on blastomere sizes or reduc-
tion in cytoplasm.
CELL SIZE CUT-OFF LIMITS
Minimum sizes for biologically competent blas-
tomeres must be defined in order to distinguish
between small blastomeres and large fragments.
Based on the presence of DNA, Johansson et al
22
suggested a cut-off size limit between blastomeres
and fragments of 45 m in diameter in day 2 embryos
and 40 m in day 3 embryos. However, this study
did not differentiate between different cleavage stages
observed on day 2 or day 3, respectively. Addition-
ally, Hnida et al
24
analyzed separate blastomeres and
found that none of the analyzed 4-cell blastomeres
smaller than 50 m in diameter contained DNA,
whereas 96% of the blastomeres with a diameter
larger than 50 m contained DNA. However, as the
diameter of the blastomeres in the intact 4-cell
embryos was approximately 3% smaller compared
with the separate blastomeres, Hnida et al suggest a
cut-off diameter of approximately 45–50 m between
blastomeres and fragments in 4-cell stage embryos.
24
EMBRYOS WITH BLASTOMERES OF UNEVEN SIZE
The impact of unequal-sized blastomeres in an
embryo has long been discussed, as this might be
part of normal embryo development, particularly in
dividing embryos (Figure 8.3). There is also no gen-
eral definition regarding how large the difference
must be in order for blastomeres to be classified as
of unequal size. However, in recent years a number of
studies have demonstrated that the developmental
potential of embryos with blastomeres of unequal
sizes is compromised. Studies have demonstrated
6,30
that implantation and pregnancy rates were both
significantly lowered after transfer of embryos with
unevenly sized blastomeres. Two studies
19,30
found a
highly significant correlation between embryos with
blastomeres having more than 25% difference in
blastomere size and chromosomal abnormality.
Further, the findings by Hardarson et al
30
indicate
that embryos with unevenly sized blastomeres have
increased rates of multinucleation and that there is
HPE_Chapter08.qxp 7/18/2007 3:08 PM Page 96
MORPHOMETRIC ANALYSIS OF HUMAN EMBRYOS
In normal cleaving cells, there is a very close
interaction and timing of the processes that
control karyokinesis and cytokinesis.
32,33
However,
other mechanisms may also be involved in the forma-
tion of multinucleated blastomeres, including errors
in chromosome migration at mitosis, incorrect pack-
aging of chromosomes by the nuclear membrane
after mitosis, or fragmentation of the nuclei.
17,22,31
Additionally, mononucleated blastomeres originat-
ing from multinucleated embryos are on average
smaller in size than the blastomeres from mono-
nucleated embryos (Figure 8.7), and the frequency of
anucleate blastomeres (by definition large fragments)
is higher in multinucleated embryos.
18,24
This indi-
cates that asymmetric cell cleavage may also be asso-
ciated with the occurrence of multinucleation.
17,18,24
In conclusion, detection of nuclear status, espe-
cially in embryos that are otherwise of good mor-
phology is of great importance in order to improve
clinical outcome. However, assessment of multinu-
cleation cannot be evaluated on the basis of blas-
tomere size alone. Detection of embryos with
unevenly sized blastomeres should be used in com-
bination with visual verification of nuclear structures.
Hnida et al
24
analyzed the nuclear status in a cohort
of embryos and showed that significantly more
embryos were correctly categorized using the
multilevel digital imaging system compared with
traditional evaluation.
SIZE OF NUCLEI
Despite the fact that nuclear : cell volume ratio is
known to control the timing of events in early embry-
onic development in other species,
34,35
very little is
known about nuclear sizes in human embryos. Using
morphometric multilevel measurements to assess the
size of the nuclei in good quality mononucleated
2-cell embryos showed a diameter of 22.1 m and a
volume of 0.006 ⫻ 10
6
m
3
. This decreased to a
diameter of 18.7 m and a volume of 0.003 ⫻ 10
6
m
3
in 4-cell embryos. These findings suggest a consis-
tent nuclear : cell volume ratio of approximately 0.2
at least up to the 4-cell stage in human embryos.
24
KINETICS OF EARLY EMBRYONIC
DEVELOPMENT
Embryo assessment traditionally consists of scor-
ing individual features such as cell number and
fragmentation. However, it is important to bear in
2-cells
3-cells
4-cells
0
0.1
0.2
0.3
0.4
Blastomere volume (µm
3
× 10
6
)
Multinucleated blastomeres
Mononucleated blastomeres
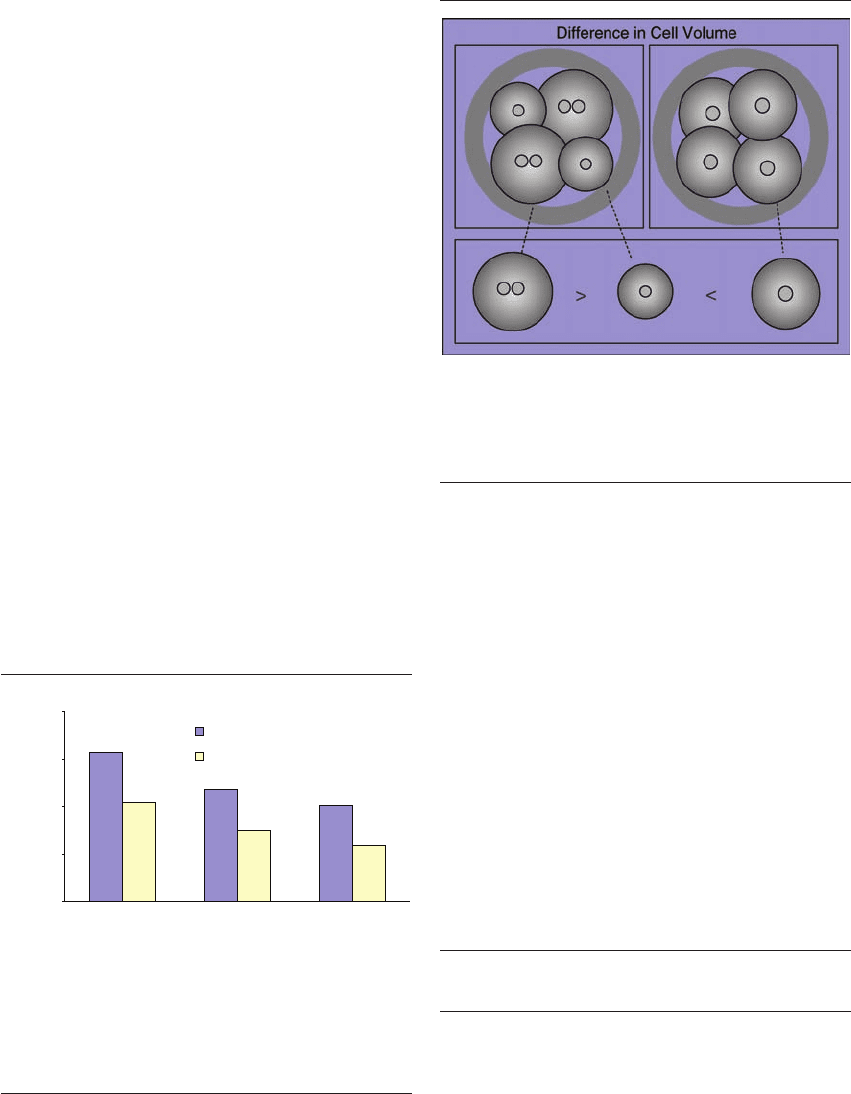
Figure 8.6 It has previously been suggested that multinucle-
ation may originate from an uncoupling of processes that con-
trol karyokineses and cytokinesis, resulting in duplication of the
nucleus without subsequent cell cleavage. These speculations are
supported by the findings that a multinucleated 4-cell blastomere
is approximately the same size as a non-multinucleated 2-cell
blastomere.
Figure 8.7 The volume of multinucleated blastomeres is signifi-
cantly larger than their mononucleated sibling blastomeres.
Further, mononucleated blastomeres from multinucleated
embryos are smaller in size than the blastomeres from
mononucleated embryos.
HPE_Chapter08.qxp 7/18/2007 3:08 PM Page 97

HUMAN PREIMPLANTATION EMBRYO SELECTION
analysis also presents many exciting possibilities.
Although this technique is still young and needs
further development, some very promising possi-
bilities are already available today.
REFERENCES
1. Puissant F, Van Rysselberge M, Barlow P et al. Embryo scoring as a
prognostic tool in IVF treatment. Hum Reprod 1987; 2: 705–8.
2. Schulman A, Ben-Num I, Gethler Y et al. Relationship between embryo
morphology and implantation rate after in vitro fertilization treatment
in conception cycles. Fertil Steril 1993; 60: 123–6.
3. Giorgetti C, Terriou P, Auquier P et al. Embryo score to predict implan-
tation after in-vitro fertilization: based on 957 single embryo transfers.
Hum Reprod 1995; 10: 2427–31.
4. Van Royen E, Mangelschots K, De Neubourg D et al. Characterization
of top quality embryo, a step towards single-embryo transfer. Hum
Reprod 1999; 14: 2345–9.
5 Hill GA, Freeman M, Bastias MC et al. The influence of oocyte matu-
rity and embryo quality on pregnancy rate in a program for in vitro
fertilization-embryo transfer. Fertil Steril 1989; 52: 801–6.
6. Ziebe S, Petersen K, Lindenberg S et al. Embryo morphology or
cleavage stage: how to select the best embryo for transfer after in vitro
fertilization. Hum Reprod 1997; 12: 1545–9.
7. Erenus M, Zouves C, Rajamahendran P et al. The effect of embryo
quality on subsequent pregnancy rates after in vitro fertilization. Fertil
Steril 1991; 56: 707–10.
8. Steer CV, Mills CL, Tan SL, Campbell S and Edwards RG. The cumula-
tive embryo score: a predictive embryo scoring technique to select the
optimal number of embryos to transfer in an in-vitro fertilization and
transfer programme. Hum Reprod 1992; 7: 117–19.
9. Van Royen E, Mangelschots K, De Noubourg D et al. Calculating the
implantation potential of day 3 embryos in woman younger than 38
years of age: a new model. Hum Reprod 2001; 16: 326–32.
10. Van Blerkom J, Davis P and Alexander S. A microscopic and biochem-
ical study of fragmentation phenotypes in stage-appropriate human
embryos. Hum Reprod 2001; 16: 719–29.
11. Hardarson T, Lofman C, Coull G et al. Internalization of cellular frag-
ments in a human embryo: time-lapse recordings. Reprod Biomed
Online 2002; 5: 36–8.
12. Tsuji K, Sowa M and Nakano R. Relationship between human oocyte
maturation and different follicular sizes. Biol Reprod 1985; 32: 413–17.
13. Goyanes VJ, Ron-Corzo A, Costas E and Maneiro E. Morphometric cat-
egorization of the human oocyte and early conceptus. Hum Reprod
1990; 5: 613–18.
14. Wolf JP, Bulwa S, Rodrigues D and Jouannet P. Human oocyte cytometry
and fertilisation rate after subzonal insemination. Zygote 1995; 3: 101–9.
15. Balakier H and Cadesky K. The frequency and developmental capabil-
ity of human embryos containing multinucleated blastomeres. Hum
Reprod 1997; 12: 800–4.
16. Roux C, Joanne C, Agnani G et al. Morphometric parameters of living
human in-vitro fertilization embryos; importance of asynchronous
division process. Hum Reprod 1995; 10: 1201–7.
17. Hardy K,Winston RML and Handyside AH. Binucleate blastomeres in
preimplantation human embryos in vitro: failure of cytokinesis dur-
ing early cleavage. J Rep Fertil 1993; 98: 549–58.
18. Hnida C, Engenheiro E, Ziebe S. Computer controlled multi-level mor-
phometric analysis of blastomere size as biomarker of fragmentation
and multinuclearity in human embryos. Hum Reprod 2004; 19: 288–93.
mind that embryo development is a dynamic process
and that the kinetics involved can yield additional
information about embryo competence.
A number of studies have demonstrated that the
timing of cell cleavage is a significant indicator of
embryonic competence. The embryo needs not only
to develop to the 4-cell stage, but also it needs to do so
at the correct time. Cleavage that occurs too rapidly
or too slowly is an indication of impaired compe-
tence. Likewise, the onset of mitoses and the appear-
ance/disappearance of the pronuclei after fertilization
need to take place during a narrow time interval
(22–25 hours) in high quality embryos, as suggested
by Fancsovits et al.
36
It has also been suggested that
the interval between pronuclear breakdown and the
first cleavage division should be relatively constant,
about 3 hours.
37,38
Other studies have demonstrated that the occur-
rence of early cleavage may be a good prognostic fac-
tor. However, the specific timing of early cleavage
seems to be related to the method of fertilization,
suggesting that different kinetics are involved in the
processes of ICSI vs regular IVF.
39
CONCLUSION
In the past, embryo evaluation has been based mainly
on subjective evaluation of morphological parame-
ters considered to be important markers of quality.
However, a number of drawbacks are associated
with this type of analysis. One example is the differ-
entiation between large fragments and blastomeres,
and another is imprecise estimation of the degree of
fragmentation. The introduction of computer-based
morphometric analysis has allowed us to enter a new
level of embryo evaluation. These techniques open
an array of possibilities for standardization and more
precise measurements, including total cytoplasmic
reduction as a new means of describing fragmenta-
tion, and detection of multinucleation based on
blastomere size.
In the final analysis, the combination of kinetics
and morphometrics that include detailed informa-
tion retrieved over several days is a new and fasci-
nating aspect. The ‘3-dimensionality’ of multilevel
HPE_Chapter08.qxp 7/18/2007 3:08 PM Page 98
MORPHOMETRIC ANALYSIS OF HUMAN EMBRYOS
19. Ziebe S, Lundin K, Loft A for the CEMAS II and III Study Group et al.
FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blas-
tomeres of IVF pre-embryos from 144 randomly selected donated
human oocytes and impact on pre-embryo morphology. Hum Reprod
2003; 18: 2575–81.
20. Ebner T,Yaman C, Moser M et al. Embryo fragmentation in vitro and its
impact on treatment pregnancy outcome. Fertil Steril 2001; 76: 281–5.
21. Alikani M, Cohen J, Tomkin G et al. Human embryo fragmentation in
vitro and its implications for pregnancy and implantation. Fertil Steril
1999; 71: 836–42.
22. Johansson M, Hardarson T, Lundin K. There is a cutoff limit in
diameter between a blastomere and a small anucleate fragment. J Assist
Reprod Genet 2003; 20: 309–13.
23. Hnida C, and Ziebe S. Total cytoplasmic volume as biomarker of frag-
mentation in human embryos. J Assist Reprod Genet 2004; 20: 335–40.
24. Hnida C, Agerholm I and Ziebe S. Traditional detection versus
computer-controlled multilevel analysis of nuclear structures from
donated embryos. Hum Reprod 2005; 20: 665–71.
25. Jackson KV, Ginsburg ES, Hornstein MD, Rein MS and Clarke RN.
Multinucleation in normal fertilized embryos is associated with an
accelerated ovulation induction response and lower implantation
rates in in vitro fertilization-embryo transfer cycles. Fertil Steril 1998;
70: 60–6.
26. Pelinck MJ, De Vos M, Dekens M et al. Embryos cultured in vitro with
multinucleated blastomeres have poor implantation potential in human
in-vitro fertilization and intracytoplasmic sperm injection. Hum
Reprod 1998; 13: 960–3.
27. Van Royen E, Mangelschots K,Vercruyssen M et al. Multinucleation in
cleavage stage embryos. Hum Reprod 2003; 18: 1062–9.
28. Kligman I, Benadiva C, Alikani M, and Munné S. The presence of
multinucleated blastomeres in human embryos is correlated with chro-
mosomal abnormalities. Hum Reprod 1996; 11: 1492–8.
29. Balakier H. and Cadesky K.The frequency and developmental capabil-
ity of human embryos containing multinucleated blastomeres. Hum
Reprod 1997; 12: 800–4.
30. Hardarson T, Hanson C, Sjögren A, and Lundin K. Human embryos
with unevenly sized blastomeres have lower pregnancy and implanta-
tion rates: indications for aneuploidy and multinucleation. Hum
Reprod 2001; 16: 313–18.
31. Pickering SJ, Taylor A, Johnson MH, and Braude PR. An analysis of
multinucleated blastomere formation in human embryos. Hum Reprod
1995; 10: 1912–22.
32. Burke B. and Ellenberg J. Remodelling the walls of the nucleus. Nat Rev
2002; 3: 487–97.
33. Straight AF. and Field CM. Microtubules, membranes and cytokinesis.
Curr Biol 2000; 10: 760–70.
34. Masui M, and Kominami T. Change in the adhesive properties of blas-
tomeres during early cleavage stages in sea urchin embryo. Dev
Growth Differ 2001; 43: 43–53.
35. Masui M, Yoneda M, and Kominami T. Nucleus : cell volume ratio
directs the timing of increase in blastomere adhesiveness in starfish
embryos. Dev Growth Differ 2001; 43: 295.
36. Fancsovits P, Toth L, Takacs Z.F et al. Early pronuclear breakdown is
a good indicator of embryo quality and viability. Fertil Steril 2005;
84: 881–7.
37. Van Wissen B, Wolf JP, Bomsel-Helmreich O, Frydman R, and Jouannet
P. Timing of pronuclear development and first cleavages in human
embryos after subzonal insemination: influence of sperm phenotype.
Hum Reprod 1995; 10: 642–848.
38. Capmany G, Taylor A, Braude PR, and Bolton VN. The timing of
pronuclear formation, DNA synthesis and cleavage in the human 1-
cell embryo. Mol Hum Reprod 1996; 2: 299–306.
39. Van Montfoort APA, Dumoulin JCM, Kester ADM, and Evers JLH
Early cleavage is a valuable addition to existing embryo selection
parameters: a study using single embryo transfers. Hum Reprod 2004;
9: 2103–8.
40. Arce J-C, Ziebe S, Lundin K et al. Interobserver agreement and intra-
observer reproducibility of embryo quality assessments. Hum Reprod
2006; 21: 2141–8.
41. Lehtonen E, et al. Changes in cell dimensions and intercellular contacts
during cleavage-stage cell cycles in mouse embryonic cells. J Embryol
Exp Morphol 1980; 58: 231–9.
42. Massip A. and Mulnard J. Time-lapse cinematographic analysis of
hatching of normal and frozen-thawed cow blastocysts. J Reprod Fertil
1980; 58: 475–8.
43. Aiken CEM, Swoboda PPL, Skepper JN. and Johnson MH. The direct
measurement of embryonic volume and nucleo-cytoplasmic ratio
during mouse pre-implantation development. Reproduction 2004;
128: 527–35.
44. Alikani M, Cohen J, Tomkin G et al. Human embryo fragmentation in
vitro and its implications for pregnancy and implantation. Fertil Steril
1999; 71: 836–42
45. Munne S, Alikani M. and Cohen J. Monospermic polyploidy and
atypical embryo morphology. Hum Reprod 1994; 9: 506–10.
HPE_Chapter08.qxp 7/18/2007 3:08 PM Page 99
HPE_Chapter08.qxp 7/18/2007 3:08 PM Page 100

9. Development rate, cumulative scoring, and
embryonic viability
Christine C Skiadas and Catherine Racowsky
standard of care, even the most rigorous selection
paradigms have limitations, including the inability
to detect genetic disorders or predict pregnancy
with 100% accuracy. Other embryonic markers of
development and metabolic assessment are cur-
rently being explored and these may, in the future,
be used alone or in combination with morphologi-
cal evaluations for improved embryo selection.
This chapter reviews the normal timeline and
sequence of embryonic development from fertiliza-
tion through progression to the blastocyst stage. We
also review the key features of morphological crite-
ria for embryo selection, cumulative grading sys-
tems and their association with implantation rates
and viability. Finally, we consider issues surround-
ing the optimum day for embryo transfer.
DEVELOPMENTAL RATE: NORMAL
TIMELINE OF EVENTS
Preimplantation development follows a programmed
timeline during which an organized series of critical
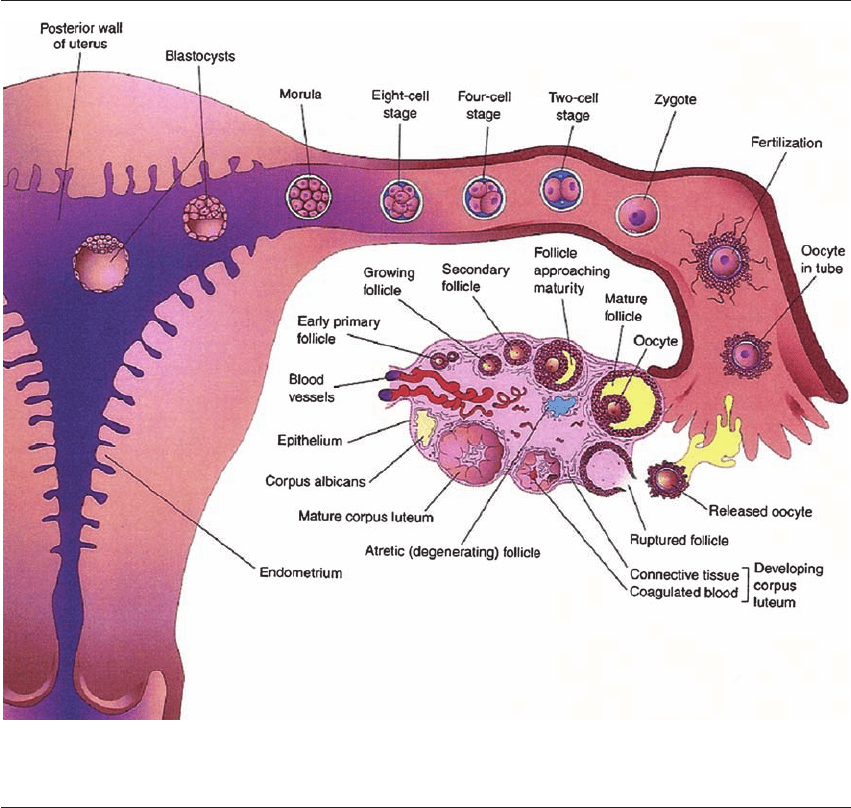
events take place (Figure 9.1). In vivo, fertilization
and early cleavage occur in the fallopian tube, with
the embryo traversing the uterotubal junction at the
morula stage. The procedure of clinical IVF has
allowed this timeline of events to be observed in
detail and researched. In terms of defining embryo
morphology, one of the first time points that has
been evaluated is that of the zygote or pronuclear
embryo,
12–15
approximately 16–18 hours after fertil-
ization. Key features of the zygote stage are the
development of the two pronuclei (one from the
oocyte and one from the sperm), each containing
multiple nuclear precursor bodies. The two pro-
nuclei (PNs) migrate towards each other and their
INTRODUCTION
Since the inception of clinical in vitro fertilization
(IVF), there has been a drive to optimize pregnancy
rates. Although this was initially achieved by trans-
ferring greater numbers of embryos, transfers of
multiple embryos resulted in the negative side-effect
of increasing the incidence of high-order multiple
gestations.
1–4
In order to reduce high-order multiple
gestations and at the same time maintain pregnancy
rates, there has been a progressive move toward
decreasing the number of day 3 embryos trans-
ferred,
5
as well as performing day 5 transfers of only
one or two blastocysts.
6–8
However, blastocyst trans-
fer may not be ideal in all cases, and may compro-
mise a successful outcome that would otherwise be
achieved following a day 3 transfer.
8–10
Therefore,
one of the most important challenges in IVF is the
ability to determine which embryos are associated
with the greatest developmental potential, in order
to select optimally only one, or at most two, of these
embryos for transfer.
Ideal methods for embryo selection include: ease
of assessment, standardization among embryologists,
minimal harm to the embryo, and a high correlation
with pregnancy rates; therefore morphological assess-
ment remains the first-line approach, although non-
invasive biomarker methods are currently under
development (Chapters 12 and 20). Since human
embryonic development follows a specifically timed,
coordinated sequence of events, developmental rate
(assessed by certain milestones being reached at
particular points in time) and morphological char-
acteristics (defined at specified intervals after the
day of insemination) provide the two main measures
of embryonic development. Although morphologi-
cal selection of embryos represents the current
HPE_Chapter09.qxp 7/13/2007 4:49 PM Page 101
HUMAN PREIMPLANTATION EMBRYO SELECTION
multiple nucleoli align at the pronuclear interface in
preparation for syngamy.
16,17
Specific features of the
PNs have been used in zygote scoring systems,
which are discussed in Chapters 3 and 4.
Following syngamy, the newly formed zygote
undergoes first cleavage between 20 and 27 hours
after insemination,
18–20
and second cleavage to form
the 4-cell stage at approximately 48 hours. The
embryo reaches the 8-cell stage by approximately
72 hours. Early cycles of cell division are thought to
be regulated by the maternal genome, with the
embryonic genome becoming activated to dictate
further cell divisions at approximately day 3, between
the 4- and 8-cell stage.
21
Following the 8-cell stage,
the cells become increasingly polarized and the
embryo develops cell–cell adhesions and gap junc-
tions during the process of compaction. This is
expected to occur on day 4,
22
with progression to
Figure 9.1 In vivo embryo maturation. Natural timeline of embryonic development in vivo. Early cleavage stages occur in the
fallopian tube and the embryo enters the uterus once it has reached the blastocyst stage. Reprinted from Figure 2-24, Moore and
Persaud, The Developing Human, 6 edn. Philadelphia: WB Sanders, 1998: 44, Copyright 1998, with permission from Elsevier.
11
HPE_Chapter09.qxp 7/13/2007 4:49 PM Page 102
DEVELOPMENT RATE, CUMULATIVE SCORING, AND EMBRYONIC VIABILITY
blastocyst development early on day 5 and comple-
tion of blastulation by late day 5.
23
Figure 9.2 shows
representative images of embryos at these stages.
EMBRYONIC MORPHOLOGY AND
SCORING SYSTEMS
The optimal timing and method of morphological
assessment has been debated amongst embryologists,
and has been affected by laws governing embryo
transfer and cryopreservation.
ZYGOTE STAGE
Zygote scoring systems allow embryos of optimal
prognosis to be identified immediately after fertil-
ization, which has been particularly useful in countries
where embryo selection at cleavage stages is restricted.
In Germany, the German embryo protection law
dictates that the number of embryos to be trans-
ferred must be selected at the PN stage, with the
remainder being cryopreserved. However, despite the
positive results and practical benefits of PN scoring,
there remains debate over whether such evaluation
is superior to that of standard morphological assess-
ment. Indeed, a recent study
24
investigated whether
PN scoring was superior to standard day 2 or day 3
morphological assessment; the results failed to
detect a difference in pregnancy rates based on the
scoring system. Although this study was underpow-
ered to conclude a negative result, it suggests that
the ideal method of scoring has yet to be deter-
mined. Zygote scoring systems, and their physiolog-
ical basis, are covered in detail in Chapters 3 and 4.
TWO CELL EMBRYOS
Although very few studies address the morphologi-
cal features of the 2-cell embryo, the time to first cell
division has been extensively studied as a predictor
of improved pregnancy outcomes. Embryos that
undergo ‘early cleavage’ have been postulated to
have a greater degree of developmental competence
than embryos that do not undergo early cleavage.
25
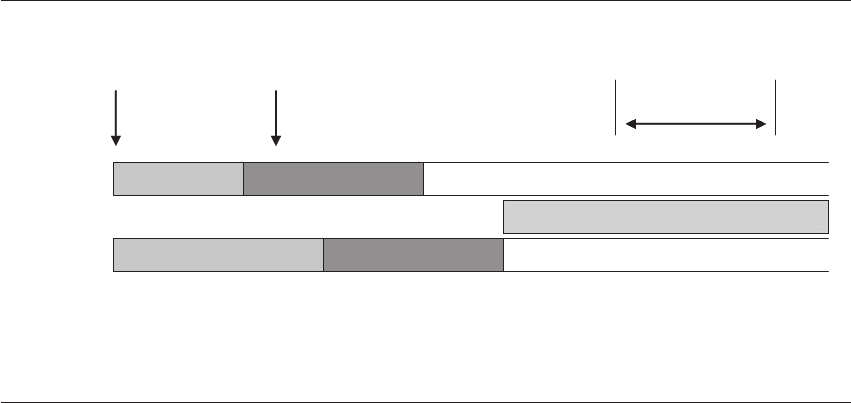
In 1997, Shoukir et al designated those embryos that
had reached the 2-cell stage by 25 hours postinsem-
ination as having undergone ‘early cleavage,’ and
this occurred in 19% of cycles (see Figure 9.3).
Cycles with early cleavage were associated with sig-
nificantly higher pregnancy rates.
26
As the timing of
fertilization is often unknown with conventional
insemination, a follow-up study using only ICSI
cycles again confirmed that those embryos under-
going early cleavage were associated with a signifi-
cantly higher pregnancy rate, supporting the idea
that early cleavage is related to developmental com-
petence and not to the timing of fertilization.
27
Tsai
et al reproduced these findings in a retrospective
study
3
and Sakkas et al confirmed their original
findings with a prospective study performed in 2001,
where the presence of an increased number of early
cleaving embryos was again associated with increased
implantation rate.
28
This study assessed early cleavage
on alternate weeks to determine if selecting embryos
on this basis had an impact on implantation rates.
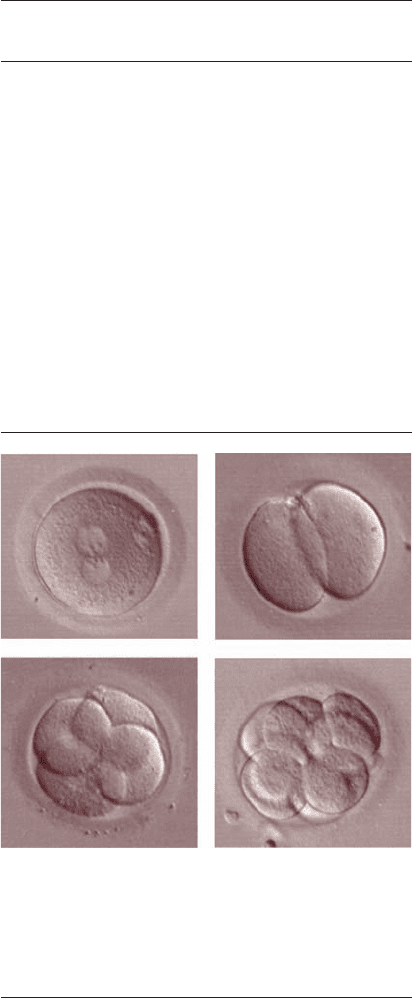
Figure 9.2 Representative embryo images. (A) Pronuclear stage
embryo. In this photograph, the two pronuclei are aligned in the
middle of the zygote with their nucleoli aligned at the pronuclear
interface. (B) 2-Cell embryo. (C) 4-Cell embryo. (D) 8-Cell
embryo. In both the 4-cell and 8-cell embryo, blastomeres
appear symmetrical and display no fragmentation.
A
B
C
D
HPE_Chapter09.qxp 7/13/2007 4:49 PM Page 103
HUMAN PREIMPLANTATION EMBRYO SELECTION
Of note, there was no difference in the total number
of embryos between groups. In the group where
early cleavage was checked, the pregnancy rate was
48%, compared with 31% in the group where this
was not checked.
28
The fact that checking for early
cleavage did improve the pregnancy rate suggests that
this may be a feature of improved developmental
competence.
In a retrospective analysis, Ciray et al showed an
association between early cleavage, a higher day 3
embryo quality score and increased implantation
rates, demonstrating that there is a link between
early cleavage and improved embryo morphology
at the 8-cell stage.
29
Therefore, if early cleavage is
another surrogate marker for improved day 3 mor-
phology and implantation, the question arises as to
whether it is necessary to evaluate the embryo at
both stages. A further discussion of multiday embryo
assessment is undertaken later in this chapter.
FOUR-CELL EMBRYOS (DAY 2)
The embryo normally reaches the 4-cell stage on
day 2 after insemination, and many of the embryo
transfers were carried out on day 2 in the earliest
reports of IVF. The features of cell number, degree
of fragmentation, and equal size of blastomeres
have been frequently evaluated at this stage to deter-
mine viability potential. Cummins et al and Puissant
et al were two of the earliest authors to describe such
scoring systems.
1,2
Ideal 4-cell embryos are those
with equal sized blastomeres and minimal fragmen-
tation. Ebner et al also confirmed that day 2
embryos displaying little fragmentation showed an
improved clinical pregnancy rate.
30
Even in these
early scoring systems, developmental rate was rec-
ognized as an additional marker of improved implan-
tation. Cummins et al reported an ideal embryo
developmental rate (EDR) associated with implan-
tation;
2
Puissant et al and Steer et al both used
embryo cell number as an approximate marker for
achieving a certain developmental state, with addi-
tional points awarded if the embryo had reached
certain milestones.
1,31
Table 9.1 summarizes studies
that focus on day 2 embryo scoring.
1,2, 31–33
Further
discussion of the individual morphological features
(cell number, fragmentation, symmetry, and com-
paction) is discussed in the section below on 8-cell
embryos.
The nuclear status of the blastomeres and the pres-
ence of mononucleation provide an additional means
of assessment in the 4-cell embryo (for additional
Early
cleavage
No early
cleavage
Insemination
25 h after
insemination
43–47 h after
insemination
2-cells
4-cells
2-cells
4-cells
No distinction between embryo stages
Transfer
Figure 9.3 Timing of assessment of early cleavage. The timeline for assessing early cleavage is crucial, as there is only a certain
window where true early cleavage can be seen. This earlier time point provides differentiation between embryos that may have similar
morphology once they reach the 4-cell stage. Reproduced from Shoukir Y et al. Early cleavage of in-vitro fertilized embryos to the
2-cell stage: a novel indicator of embryo quality and viability. Hum Reprod 1997; 12(7): 1531–6. © European Society of Human
Reproduction and Embryology, with permission from Oxford University Press/Human Reproduction.
26
HPE_Chapter09.qxp 7/13/2007 4:49 PM Page 104

DEVELOPMENT RATE, CUMULATIVE SCORING, AND EMBRYONIC VIABILITY
Table 9.1 Compilation of scoring systems assessing day 2 morphological features
Cummins et al, 1986
2
Puissant et al, 1987
1
Steer et al, 1992
31
Roseboom et al, 1995
32
Giorgetti et al, 1995
33
Title of study Embryo Quality (EQ) Embryo The Cumulative Embryo Average Morphology
Development Rating (EDR) Score (CES) Score (AMS)
Type of study Retrospective analysis Retrospective analysis Retrospective analysis Retrospective multivariate Retrospective analysis of
logistic regression single embryo transfers
Timing of embryo Multiple assessments of growth Day 2 Day 2 Day 2 Day 2
assessment rate. Grading performed on day
of transfer
Assessment of EDR: based on growth rates of An additional two points CES was calculated by NA NA
developmental high EQ embryos. Calculated were added if the embryo multiplying grade of
timing a ratio of time observed/time had reached the 4-cell stage embryo ⫻ cell number
expected to predict optimal rate by 48 hours and then summated the
of development scores of all embryos
transferred
Day 2 features EQ Scoring system (Grade 1–4) Grade 4: embryos with clear, Grade 4: equal sized Number of blastomeres, A point scale was
assessed regularity or symmetry regular blastomeres with no blastomeres symmetry of blastomeres, established, which
of blastomeres, presence of fragmentation or a Grade 3: uneven percentage of extracellular assigned a point to
fragments, and quality of maximum of 5 small blastomeres with ⬍10% fragmentation. Symmetrical each feature:
cytoplasm. anucleate fragments fragmentation blastomeres ⫽ 2 points. 1) Achieved cleavage
Ideal being grade 4 embryos: Grade 3: embryos with Grade 2: 10–50% Embryo morphology state
regular blastomeres, no unequal blastomeres, few fragmentation score ⫽ symmetry score/ 2) No fragmentation, or
fragments and clear cytoplasm or no fragments Grade 1: ⬎50% (1 ⫹ % fragmentation). ⬍20% fragmentation
(no granularity) Grade 2: fragments ⬍1/3 of fragmentation Average morphology score 3) No irregular
embryo surface ⫽ embryo morphology blastomeres
Grade 1: fragments over score/# transferred embryos 4) 4-cell stage
⬎1/3 of embryo surface
Day of transfer Did not choose embryos to Day 2 Day 2 Day 2 Day 2
transfer based on scoring
system
Outcome: Both optimal EDR and EQ ‘Good embryos’ scored Pregnancy rates rose as The odds ratio of AMS Pregnancy rate
pregnancy rates were significantly associated 5 or 6 and the number of CES rose to 42. Above was 1.63 (0.990–2.7). increased with each
with pregnancy rates ‘good embryos’ transferred this level, there was no Probability of pregnancy additional point
was significantly associated further increase in increased by 63% when (increase in pregnancy
with pregnancy rates pregnancy rates, but the AMS increased by one rate approx 4% per
only an increase in point. However, this point)
multiple gestation odds ratio crosses one.
rates The AMS was included
in the model, as it
improved the goodness of fit
of the model
NA, not applicable.
HPE_Chapter09.qxp 7/13/2007 4:49 PM Page 105
