Day A. Mining chemicals hand book
Подождите немного. Документ загружается.


© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
Flotation of sulfide ores
109
AAEERROOFFLLOOAATT 224422 pprroommootteerr
– This is the ammonium salt of
AEROFLOAT 31 promoter. It is water soluble, but should be made
up at minimum 10% strength to avoid precipitation of the second-
ary collector. Widely used for flotation of Pb from Pb/Zn ores and
Cu/Pb from Cu/Pb/Zn ores. Improves Ag recovery from these ores.
AAEERROO 77331100 pprroommootteerr
– This is similar to AEROFLOAT 241 promoter
but with a higher activity.
Comments
•
AAEERROOFFLLOOAATT 2255
and
3311
promoters have considerable frothing
properties, much more so than their ammonium salts,
AAEERROOFFLLOOAATT 224411
and
224422
promoters
..
• In alkaline circuit, the aryl AEROFLOAT promoters have a much
lower tendency than xanthates to float pyrite, pyrrhotite, and
unactivated sphalerite.
• Unlike xanthates, the aryl AEROFLOAT promoters are stable in
acid circuit; however, lose their selectivity against iron sulfides.
Consequently,
AAEERROOFFLLOOAATT 2255
and
3311
promoters can be used as
strong, non-selective sulfide promoters for bulk flotation in
acid circuit.
•
AAEERROOFFLLOOAATT 2255
and
3311
promoters should be added to the pulp
full strength. Because they are in the free acid form, pre-mixing
with water or
AAEERROOFFLLOOAATT 224411
or
224422
promoters, or any other
aqueous product could release toxic H
2
S gas. This precaution
does not apply to the addition of these reagents to pulps in the
amounts normally used for flotation.
Physical characteristics
AEROFLOAT Viscosity (cps)
promoters Color S.G. 25°C**
25 Dk. Brown --- Blk. 1.19 100-200
31 Dk. Brown --- Blk. 1.19 250-500
241* Dk. Brown --- Blk. 1.13 300-800
242* Dk. Brown --- Blk. 1.13 300-600
7310 Yellow --- Brown 1.14 80-100
**Water Soluble -- Solution strength of AEROFLOAT 242 promoter should never be
less than 10%.
**Brookfield Model LVF No.2 spindle, 30rpm

© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
Mining Chemicals Handbook
110
A.2 Monothiophosphates
AERO 5688 promoter is a novel collector based on monothiophos-
phate chemistry. In commercial use at a number of operating
locations around the world, AERO 5688 promoter is particularly
effective for selective flotation of precious metals in alkaline circuits
(pH > 7.0). It is also effective in the flotation of sulfide minerals and
precious metals in acid circuits. In moderately alkaline circuits
(pH 7-10), it can be used for selective flotation of copper sulfide
minerals and precious metals from ores in which the presence of
highly activated iron sulfide minerals precludes the use of other
sulfide collectors; in fact with respect to iron sulfides, AERO 5688
promoter is one of the most selective of the available sulfide
collectors in alkaline circuits.
Typical properties AERO 5688 promoter
Appearance Clear amber to red liquid
Specific Gravity, @ 20°C (68°F) 1.20
pH >13
Viscosity, Brookfield LVT,
cps @ 20°C (68°F) 15-35
Spindle#2 @ 60 rpm
Freezing Point
Crystallization begins, °C (°F) 2 (36)
Pourable Slurry forms, °C (°F) -10 (14)
Product Solidifies, °C (°F) -16 (3)
Freeze-thaw Stability Good
Conductivity (µmhos) 23.6-24
Solubility in Water Infinite
Comments/Primarily used in the flotation of:
• Base metal sulfides, gold/silver and PGMs from ores in acid
circuit (pH 3-7).
• Selective gold/silver and copper sulfides flotation in mildly
alkaline circuits (pH 7-10).
• Used in conjunction with traditional sulfide collectors to improve
precious metals recovery in alkaline circuits.
• Flotation of cement copper in LPF process.
• In acid circuits, dosage requirements for
AAEERROO 55668888
promoter
are significantly lower than those for the more traditional sulfide
collectors. Experience also indicates that these collectors improve
flotation kinetics, especially of slow floating gold particles.
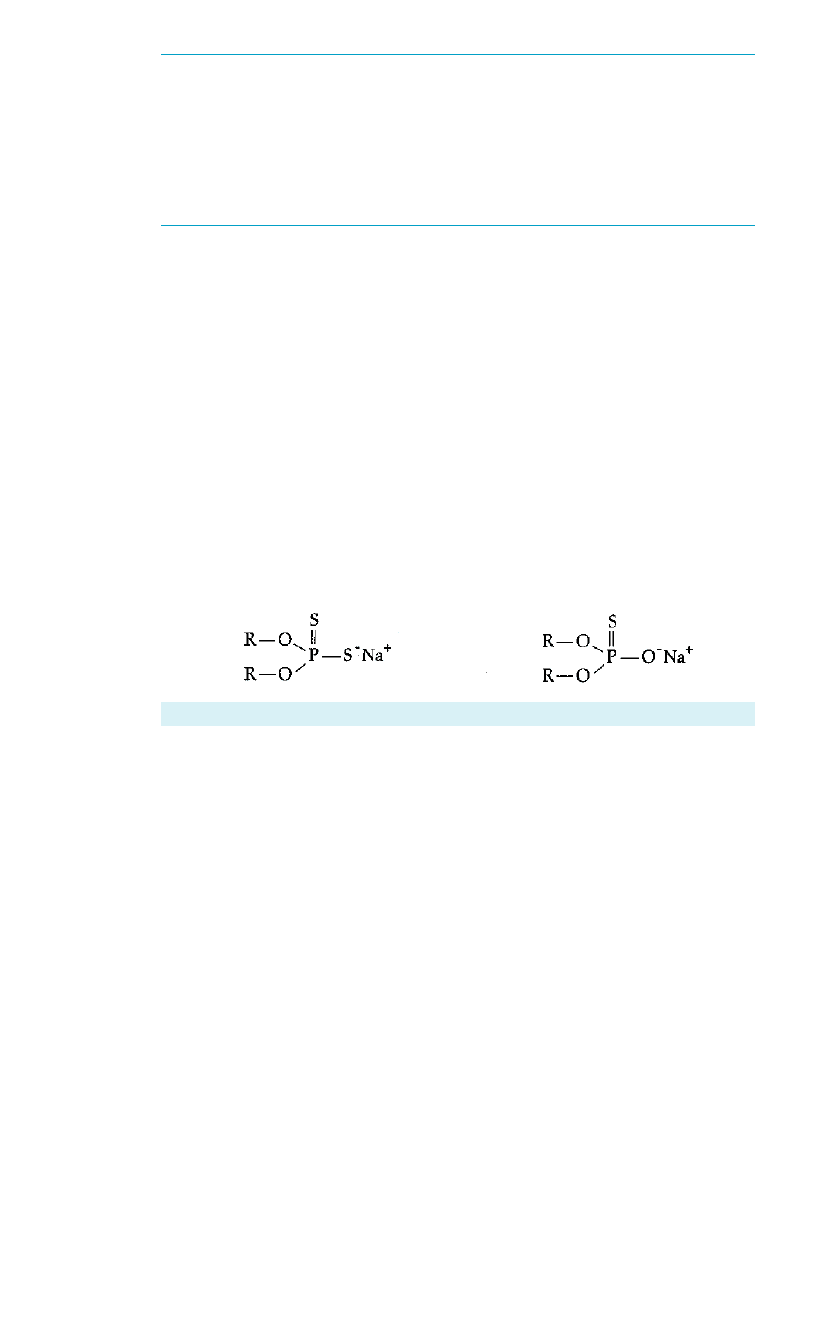
© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
• Dosage rates are usually in the range of 5 to 50 g/t for base metal
sulfide ores and up to 100 g/t for precious metal ores.
•
AAEERROO 55668888
promoter can be fed directly to the circuit, or can be
diluted with water to any strength. For ease of metering, it is often
diluted to 5-10 % strength.
•
AAEERROO 55668888
promoter exhibits some frothing properties.
A.3 Formulated P-based product
AERO 8985 promoter is a formulated product that is used for Cu-Au
Ores, where it provides optimum recovery of both Cu and Au by
combining the advantages of dithiophosphates and monothiophos-
phates.
B. Alkyl AEROFLOAT and AERO promoters
B.1 Dithiophosphates
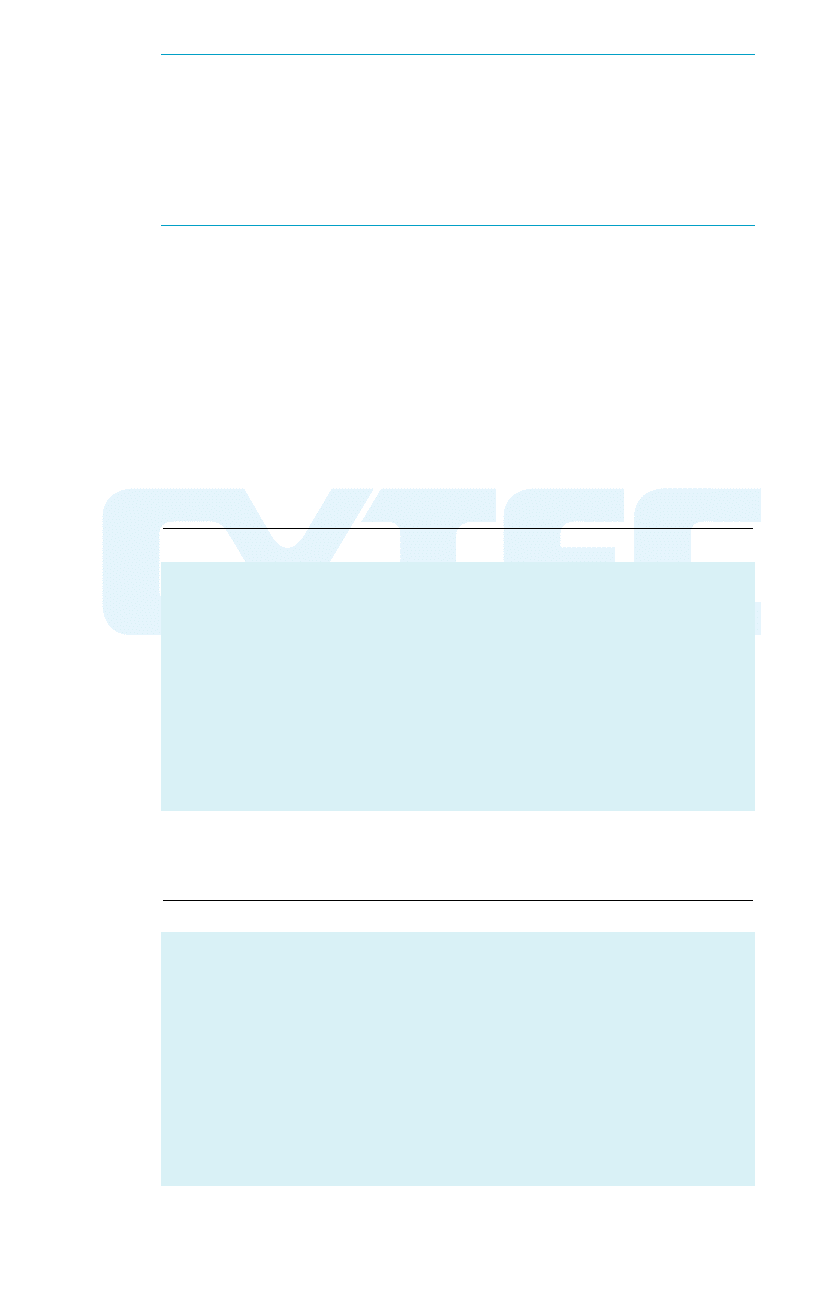
Sodium
AAEERROOFFLLOOAATT
promoter – (R=ethyl). Used mainly for selec-
tive flotation of Cu from Cu/Zn ores where Zn minerals tend to
float readily; for flotation of activated Zn sulfides where selectivity
against iron sulfides presents a problem. Very selective against iron
sulfides.
AAEERROOFFLLOOAATT 220088 pprroommootteerr
– (R=ethyl + sec. Butyl). Selective col-
lector for copper ores. Excellent collector for native Au, Ag and Cu.
AAEERROOFFLLOOAATT 221111 pprroommootteerr
– (R=isopropyl). Selective collector for
Cu and activated Zn minerals. Stronger collector than Sodium
AEROFLOAT promoter.
AAEERROOFFLLOOAATT 223388 pprroommootteerr
– (R=sec. Butyl). Widely used in Cu
flotation and for increasing by-product Au recovery. Combines good
collecting power with good selectivity against iron sulfides.
AAEERROO 33447777 pprroommootteerr
– (R= isobutyl). A strong, but selective collector
for Cu, Ni and activated Zn minerals. Improves recoveries of
precious metals, particularly those of the platinum group metals.
Flotation of sulfide ores
111
Dialkyl Dithiophosphate Dialkyl Monothiophosphate
© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
Mining Chemicals Handbook
112
AAEERROO 33550011 pprroommootteerr
– (R=isoamyl). Used for flotation of Cu and
activated Zn minerals, especially for coarse middlings. Applications
are similar to those of AERO 3477 promoter, but tends to generate
more froth.
AAEERROO 55443300 pprroommootteerr
– (R=isobutyl). A "low-frothing" version of
AERO 3477 promoter. Used when maximum froth control is desired.
AAEERROO 55447744 pprroommootteerr
– (R=isoamyl). A "low-frothing" version of
AERO 3501 promoter. Also used when maximum froth control is
desired.
Physical properties
AEROFLOAT promoters Sodium 208 211 238
Appearance Colorless to yellow liquids
pH 13.0 - 13.7
sp.gr., 30°C 1.20 1.15 1.15 1.12
Viscosity (cps)
0°C 22 25 31 45
30°C 6 7 8 12
Boiling Point, °C 103 103 103 103
Crystallization Starts, °C -4 -12 -10 -12
Pourable Slurry Forms, °C -9 -15 -10 -13
Solidification, °C -13 -29 -20 -26
Freeze-Thaw Stability Good
Physical properties
AERO promoters 3477 3501 5430 5474
Appearance Colorless to yellow liquids
pH 13.0 - 13.7
sp.gr., 30°C 1.12 1.08 1.07 1.05
Viscosity (cps)
0°C 41 38 2000 2200
30°C 11 10 750 550
Boiling Point, °C 103 103 107 107
Crystallization Starts, °C 2 4 <-20 <-20
Pourable Slurry Forms, °C -13 -4 - -
Solidification, °C -25 -9 - -
Freeze-Thaw Stability Good

© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
Flotation of sulfide ores
113
Comments
• The alkyl AERO and AEROFLOAT promoters are more selective
against iron sulfides in alkaline circuit than the corresponding
xanthates.
•
SSooddiiuumm AAEERROOFFLLOOAATT
and
AAEERROOFFLLOOAATT 220088,, 221111
and
223388
have
minimal effect upon froth generation.
•
SSooddiiuumm AAEERROOFFLLOOAATT
and
AAEERROOFFLLOOAATT 220088,, 221111
and
223388
are
poor collectors for galena, making them the ideal choice for
selective flotation of Cu from Pb.
• For many ores, the alkyl AERO and AEROFLOAT promoters are
used as the principal collector, in conjunction with a xanthate as
a secondary or scavenger collector. The longer chain ones are,
however, often used as the sole collector to insure maximum
selectivity.
B.2 Monothiophosphates
AERO 6697 promoter is a novel collector based on monothiophos-
phate chemistry, similar to AERO 5688 promoter in many of its
collector properties. AERO 6697 promoter is in commercial use at a
number of operating locations around the world. The choice between
AERO 5688 and AERO 6697 promoters depends on the mineralogy/
ore type, gangue mineralization, and frothing characteristics. On any
particular ore, both products should be tested. For a description of
typical applications, refer to Section on AERO 5688 promoters.
Physical properties
AERO 6697 promoter
Appearance Clear yellow to amber liquid
Specific Gravity, @ 20°C (68°F) 1.14
pH >13
Viscosity, Brookfield LVT,
cps @ 20°C (68°F) 15-35
Spindle#2 @ 60 rpm
Freezing Point
Crystallization begins, °C (°F) 2 (36)
Pourable Slurry forms, °C (°F) -10 (14)
Product Solidifies, °C (°F) -16 (3)
Freeze-thaw Stability Good
Solubility in Water Infinite
© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
B.3 Formulated P-based product
AERO 7249 promoter is a formulated product that is used extensively
in many Cu-Au plants, where it provides optimum recovery of both
Cu and Au by combining the advantages of dithiophosphates and
monothiophosphates, and provides excellent selectivity against iron
sulfides.
C. Dialkyl dithiophosphinates
AEROPHINE 3418A
AEROPHINE 3418A promoter is a unique, P-based sulfide collector.
It was originally developed for the flotation of copper and activated
zinc minerals. It has since been found to be an invaluable (and often
irreplaceable) collector in the beneficiation of complex, polymetallic,
and massive sulfide ores. On these ores it provides very selective
separations. It is highly effective for galena and precious metals,
especially silver. Its main attributes are strong collecting power but
with excellent selectivity against iron sulfide minerals, unactivated
sphalerite and penalty elements. On many ores, the dosage required
may be considerably lower than that needed for traditionally-used
non-selective collectors such as xanthates. Other characteristics
include:
• Low frothing contribution, even on ores containing clay minerals.
• Fast kinetics.
• Good collection of coarse middling particles.
• Excellent collector for precious metals, PGM, galena, and copper
sulfides from complex, polymetallic or massive sulfide ores.
AERO 6931 and Reagents S-4604 and S-7583 promoters
These collectors were developed recently as lower-cost versions of
AEROPHINE 3418A promoter. Comparative testing should always
be conducted, to ensure that metallurgical results are equivalent to
those obtained with AEROPHINE 3418A promoter.
Mining Chemicals Handbook
114
© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
Flotation of sulfide ores
115
6.1.4 The 400 series of AERO promoters
AAEERROO 440000 pprroommootteerr
– Used mainly for flotation of gold-bearing
pyrite in acid and neutral circuits.
AAEERROO 440044 pprroommootteerr
– Widely used for the flotation of tarnished
and secondary Cu minerals, tarnished Pb and Zn minerals, and
precious metals in alkaline circuit. Excellent collector for pyrite and
auriferous pyrite in acid and neutral circuits.
AAEERROO 440077 pprroommootteerr
– A stronger collector than AERO 404 promoter.
May substantially replace xanthates in many applications, while
being more selective against iron sulfides in alkaline circuit.
Useful for treating a wide range of precious and base-metal ores,
particularly those of Cu, Ni and Zn. Excellent for bulk flotation of
poly-metallic ores and pyritic gold ores in acid circuits.
AAEERROO 441122 pprroommootteerr
– A stronger collector than AERO 407 promoter
with substantially the same applications.
Physical properties
AERO promoters Aerofloat pro400 404 407 412
Appearance Colorless to Yellow Liquid
Boiling Point, °C 103 104 103 103
Freezing Point, ºC N/A -2 -7 9
pH >12 11.5 - 13.0
sp.gr., 25°C 1.26 1.15 1.17 1.16
Viscosity (cps)
0°C N/A 21 20 –
30°C N/A 6 6 7
Solubility Completely Water Soluble
Comments
• Generally stronger collectors than the corresponding alkyl AERO
and AEROFLOAT promoters, but still more selective than xan-
thates against iron sulfides in alkaline circuit. Use of xanthate as a
secondary collector is sometimes helpful in providing maximum
recovery.
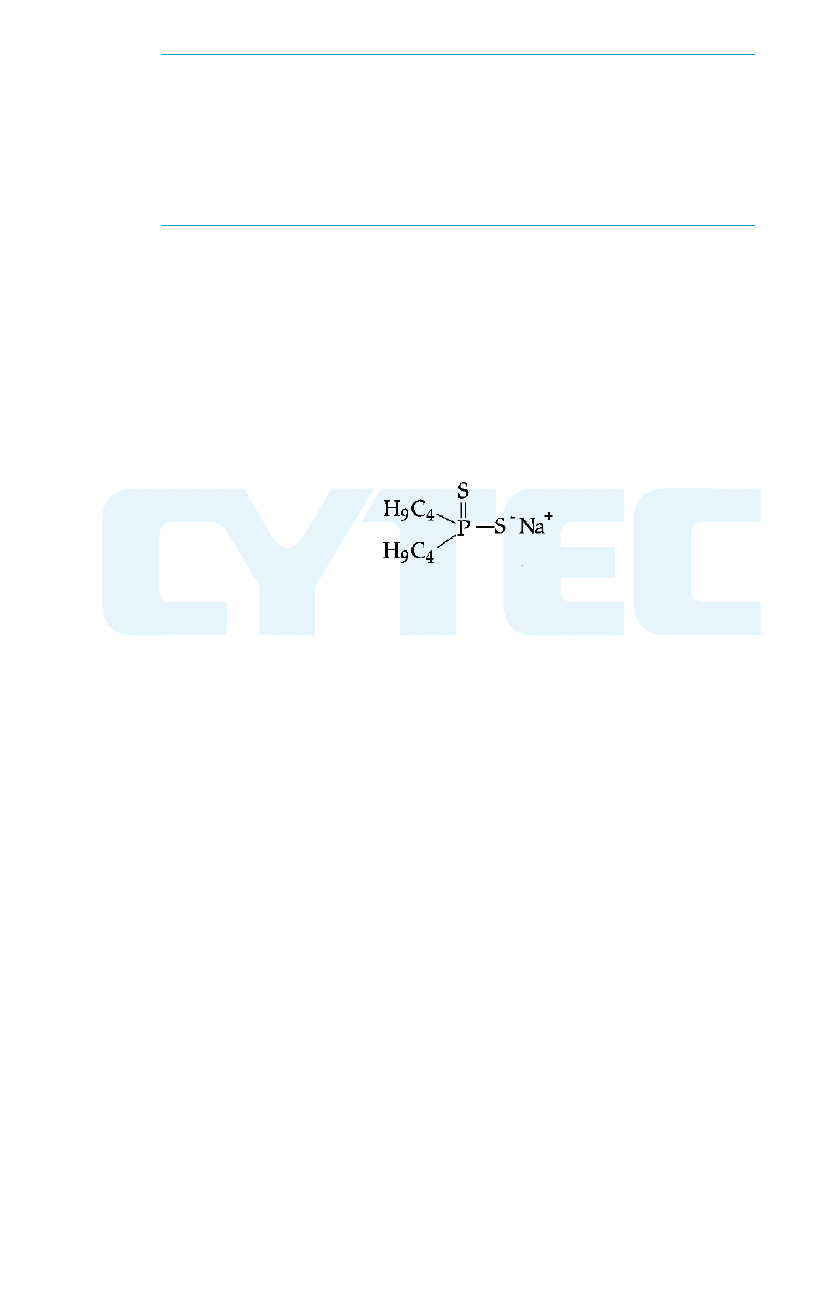
Mercaptobenzothiazole Dithiophosphate
N/A= Not Applicable
© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
• Compared to alkyl dithiophosphates, longer conditioning times
or addition to grinding mill is sometimes beneficial.
• Although originally developed mainly for the flotation of tarnished
Pb ores, the 400 series of AERO promoters are now widely used
in the flotation of most base-metal and precious metal ores. For
the flotation of "oxide" Cu, Pb and Zn minerals, pre-sulfidization
is usually required.
6.1.5 Nitrogen-based collectors
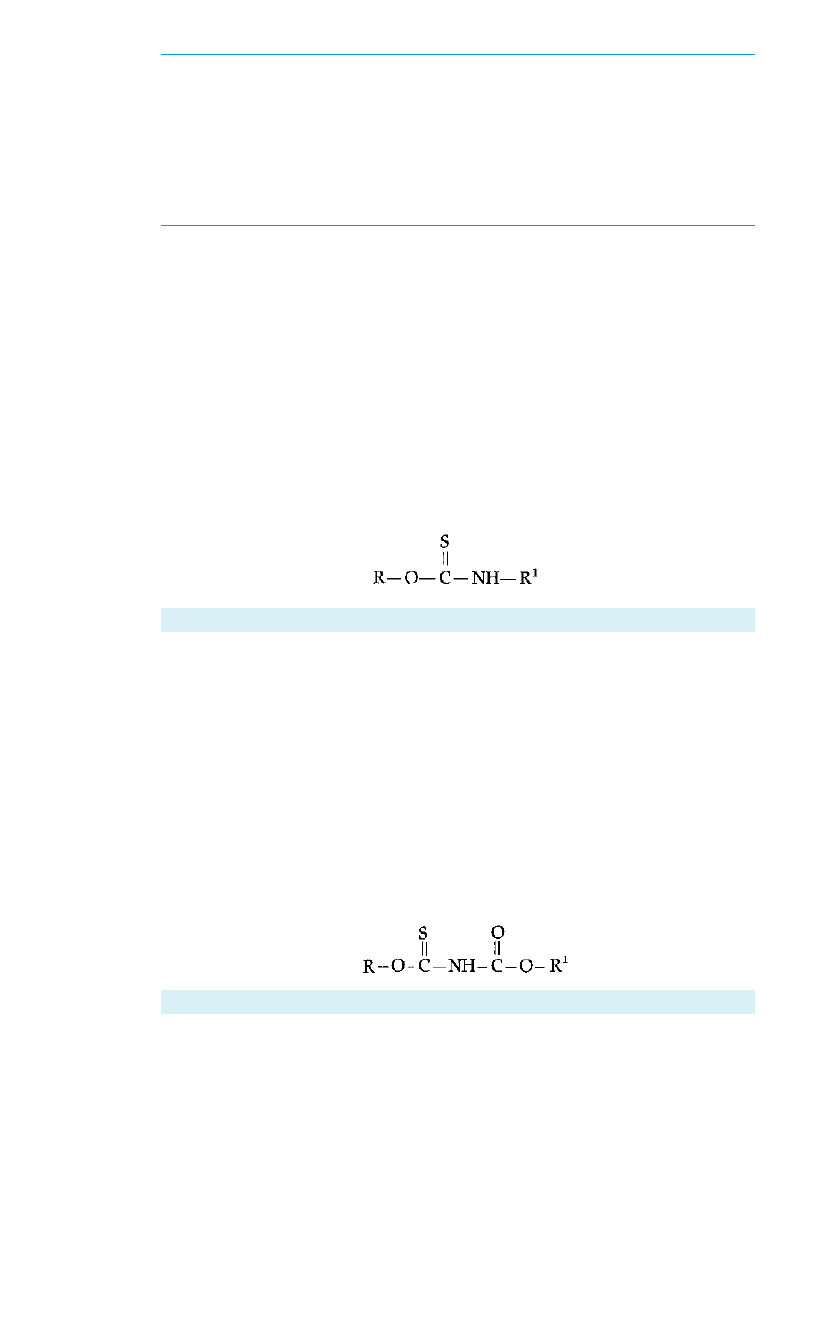
A. Dialkyl thionocarbamates
AERO 3894 promoter
This oily collector was originally developed for, and is still used in,
the selective flotation of copper ores in alkaline circuits. However,
due to its high selectivity, it generally requires the conjoint use of
a xanthate to insure maximum recovery of middling (composite)
particles. Being water-insoluble, addition to the grinding circuit is
often beneficial.
B. The Functionalized Thionocarbamates
In view of the limitations of the dialkyl thionocarbamates mentioned
above, Cytec in the mid 1980’s developed a series of funtionalized
thionocarbamates with the intention of producing collectors that
combine the selectivity of the dialkyl thionocarbamates and the
collecting power of xanthates. The other objective was to develop
collectors which would allow selective flotation of copper ores
containing iron sulfides under mildly alkaline conditions (pH 8-10)
Dialkyl Thionocarbamate
Alkyl Alkoxycarbonyl Thionocarbamate
Mining Chemicals Handbook
116

© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
in contrast to the higher pH values required to depress pyrite when
using xanthate and other collectors. Essentially this was achieved by
the incorporation in the collector molecule of an O-containing
(ethoxycarbonyl) functional group, thereby augmenting the role of
the S functional group. The introduction of this second functional
group lowers the pKa of the molecule by several orders of magni-
tude compared to that of dialkyl thionocarbamates. This allows the
collector to be effective at lower pH values. (for further discussion,
see Section 5) Further, the second functional group provides for the
formation of more favorable and stronger metal complexes and,
therefore, stronger adsorption. This has been demonstrated by
sequential adsorption studies. For example, AERO 5415 and AERO
5460 promoters have been shown to replace previously adsorbed
dialkyl thionocarbamate from the mineral surface but, on the other
hand, dialkyl thionocarbamate does not replace previously adsorbed
AERO 5415 or AERO 5460 promoters. They are especially effective
for copper-rich minerals such as chalcocite, digenite, covellite and
bornite. They are poor galena collectors, as all thionocarbamates are.
AERO 5415, AERO 5460 promoters
These two collectors are structurally similar, but AERO 5460 promoter
being the higher homologue is the more powerful of the two and,
therefore, especially suitable for the recovery of coarse middlings
particles, whilst being only slightly less selective. Both of these
collectors are now in wide commercial use (both as-is or as compo-
nents of customized formulations) for the flotation of Cu, Cu-Mo
and Cu-Au ores. In most cases, the dosage required of these collectors
is lower than that for the traditional collectors, in addition to pro-
viding considerable savings in lime costs.
Comments
• Being insoluble in water, addition to the grinding circuit or a
conditioning step ahead of flotation may be beneficial. However, in
many cases
AAEERROO 55441155
and
AAEERROO 55446600
promoters are more
readily dispersible than the dialkyl thionocarbamates and allyl
alkyl thionocarbamates (depending upon pH and other condi-
tions). Consequently, in many cases, addition to the head of flota-
tion is possible and indeed may be preferable. The best point of
addition should be determined by laboratory and plant testing.
• Because of their high collecting power in moderately alkaline
circuits, and their high selectivity against iron sulfide minerals,
Flotation of sulfide ores
117

© 1976, 1989, 2002 Cytec Industries Inc. All Rights Reserved.
the preferred rougher flotation pH for these collectors is usually
in the range of 8 to 10, compared to the typical range of 10 to 12
required with other collectors. Similarly, in the cleaner circuits,
the pH required is lower than that necessary with other collectors.
• Operating in the lower pH range not only provides a considerable
reduction in lime costs but, on ores containing significant amounts
of clay and other slimes, also reduces pulp viscosity. This usually
enhances flotation efficiency or permits operating the circuit at
higher % solids.
• It has been well established in practice that the use of
AAEERROO 55441155
and
55446600
promoters generally enhances the recovery of precious
metals.
• They are stable hydrolytically in a wide pH range.
C. Allyl Alkyl Thionocarbamates
AERO 5100 promoter
AERO 5100 promoter is a modified version of IPETC, with incorpo-
ration of an allyl group attached to the nitrogen, which increases its
collecting power but retains its known selectivity against iron sulfide
minerals. Due to its very low solubility in water, it sometimes has a
flattening effect on the froth, especially if overdosed. The optimum
point of addition – to the grind, to a conditioner, or staged-addition
– should always be determined by experiment. If a flat, dry froth is
still a problem, the conjoint use of a small amount (10% to 20% of
the AERO 5100 dosage) of a short-chain dithiophosphate such as
Sodium AEROFLOAT or AEROFLOAT 208 promoter, is often helpful.
The principal uses of AERO 5100 promoter are in the flotation of
copper, activated zinc, and precious metals. It is an extremely poor
collector for galena and is therefore an excellent choice for floating
ores which contain only nuisance amounts of lead, or for selective
flotation of copper in Cu-Pb-Zn ores.
Allyl Alkyl Thionocarbamate
Mining Chemicals Handbook
118
