Davim J.P. Tribology for Engineers: A Practical Guide
Подождите немного. Документ загружается.

219
Tribology in manufacturing
applying solid lubricants is to incorporate them within a
synthetic resin binder and to paint or spray them onto a
surface. Some of the formulations cure in air, but others
require oven drying and curing. According to Lancaster
(1984), several common constituents of bonded-fi lm lubricants
are MoS
2
, WS
2
, graphite, PTFE, pthalocyanine, CaF
2
/BaF
2
,
Pb, PbO, PbS, Sb
2
O
3
, Au, Ag, and In. Sonntag (1960) has
tabulated data for the static and kinetic friction coeffi cient of
solid lubricants on metals, and indicated whether or not they
exhibited tendencies for stick-slip. A selection of these data is
provided in Table 5.16. Since the conditions of use vary greatly
and may differ signifi cantly from Sonntag’s testing conditions,
these values are only provided as an example of relative
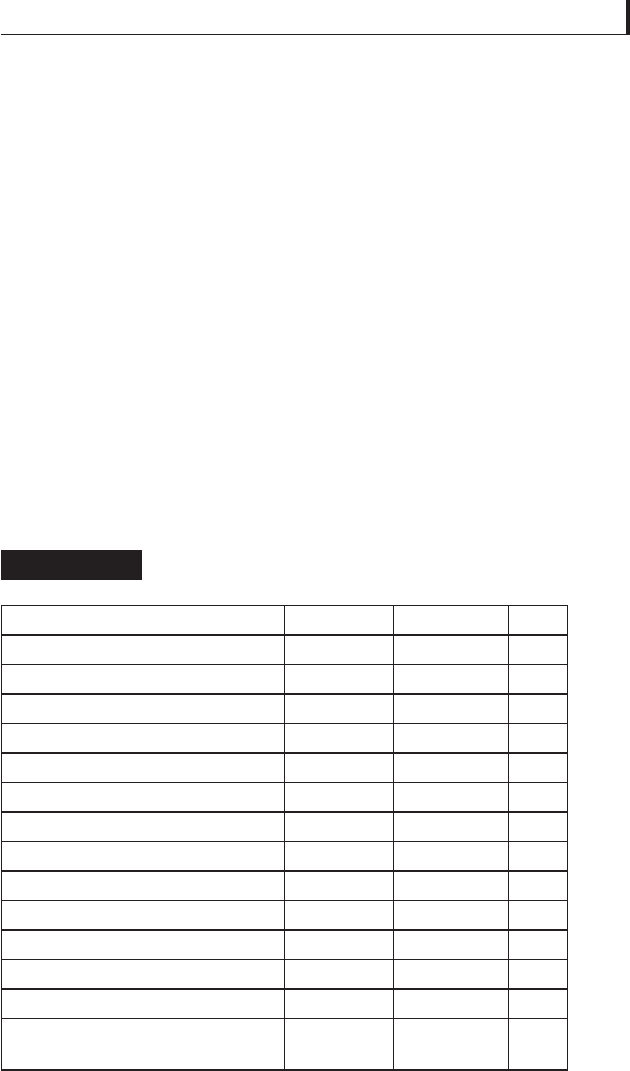
Lubricant
μ
s
μ
k
S-S
a
None (steel-on-steel) 0.40 – 0.80 0.40
Molybdenum disulfi de 0.05 – 0.11 0.05 – 0.093 N
Tungsten disulfi de 0.098 0.09 N
Selenium disulfi de, titanium — 0.25 Y
Mica, talc — 0.25 N
Graphite — 0.25 N
Boron nitride (hexagonal) — 0.25 Y
Vermiculite 0.167 0.160 N
Beeswax (at 60–63°C) 0.055 0.05 N
Paraffi n (at 47–77°C) 0.112 0.104 N
Calcium stearate (157–163°C) 0.113 0.107 N
Carnauba wax (83–86°C) 0.169 0.143 N
Sodium stearate (198–210°C) 0.192 0.164 Y
Lithium 12-hydroxystearate
(210–215°C)
0.218 0.211 N
a
S-S, tendency for stick-slip; Y, yes; N, no.
Friction coeffi cients for steel lubricated by solid
lubricants
Table 5.16
220
Tribology for Engineers
differences in the solid lubricating behaviour of various
materials at room temperature.
Solid lubricants are also used at elevated temperatures or in
high vacuum applications where most liquid lubricants would
volatilize, oxidize, or otherwise become unstable. Table 5.17
lists the friction coeffi cients of several candidate solid
lubricants obtained under the same high-temperature sliding
conditions (7.7 kgf and 7.6 mm/sec on steel at 704°C), as
reported by Peterson et al. (1969). A later compilation of
high-temperature solid lubricant friction coeffi cients was
produced by Allam (1991). Table 5.18 provides room-
temperature friction values of compressed powder pellets
sliding on stainless steel from a compilation by Clauss (1972).
Many of the most important solid lubricating materials
exhibit what has been called lamellar behaviour. That is,
there tend to be weak shear planes within the structure of the
material that can yield preferentially to reduce friction, if
they are properly aligned to the sliding direction. Many of
the compounds in Table 5.18 form hexagonal crystal
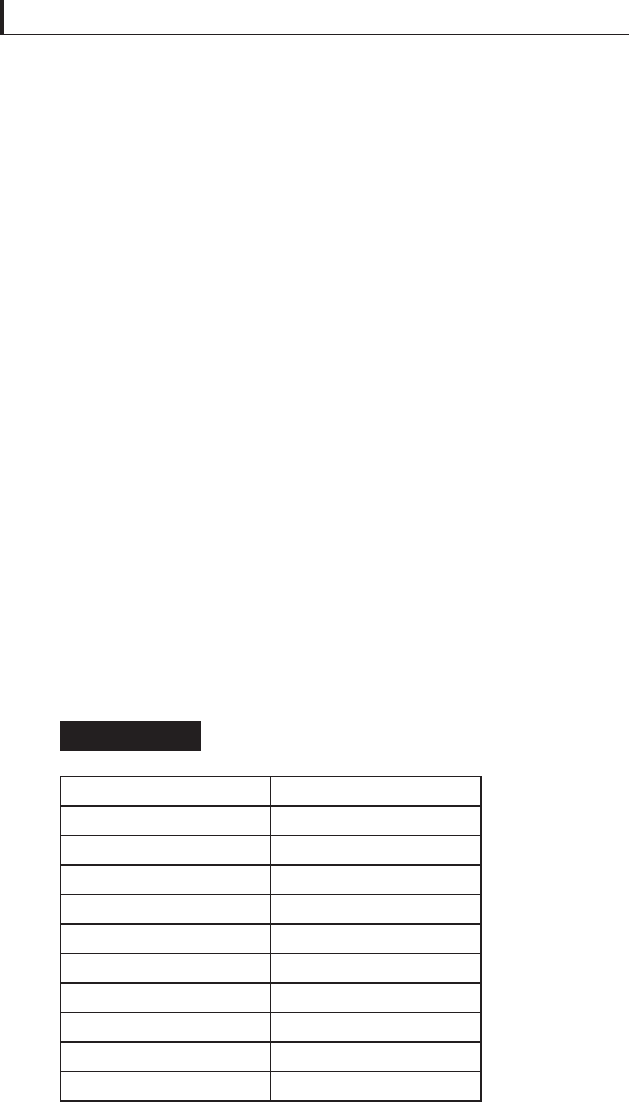
Kinetic friction coeffi cients for
several oxides at 704°C
Table 5.17
Lubricating solid
μ
PbO 0.12
B
2
O
3
0.14
MoO
3
0.20
Co
2
O
3
0.28
Cu
2
O 0.44
SnO 0.42
TiO
2
0.50
MnO
2
0.41
K
2
MoO
4
0.20
Na
2
WO
4
0.17
221
Tribology in manufacturing
structures in which the shear strength is lowest parallel to the
basal planes. In the case of molybdenum disulfi de, there are
weak van der Waals’ bonds between covalently bonded
Mo–S layers. Moisture and air tend to reduce the effectiveness
of MoS
2
as a solid lubricant, since they penetrate these layers
and raise their shear stresses. On the other hand, graphite is
observed to be more lubricious in moist environments, since
interlayer species reduce the shear strength. Therefore,
molybdenum disulfi de is very effective in low vacuum (space)
applications, but graphite is more effective in moist
environments. Table 5.19 illustrates some of these effects,
but it should be noted that solid lubricants used in powdered
form might produce different effects on friction than the
same compositions applied by other methods.
The friction of solid lubricants is sometimes modelled by
assuming that the friction force F in a given sliding system is
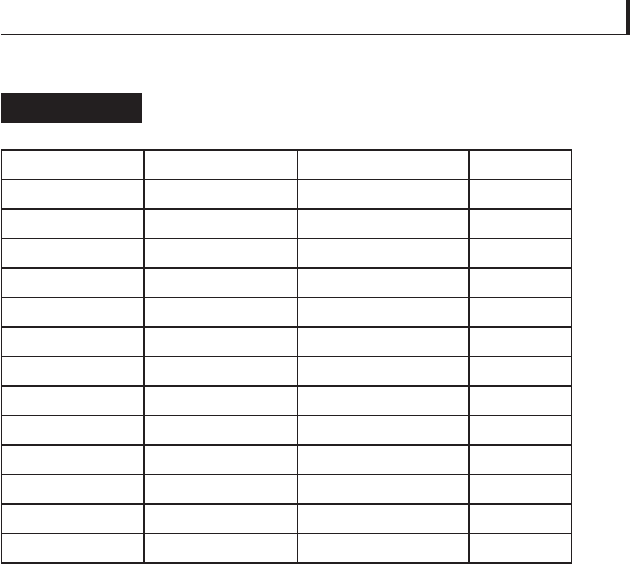
Properties and friction coeffi cients
characteristic of certain compounds
Table 5.18
Class Compound Crystal structure
Disulfi des MoS
2
Hexagonal 0.21
WS
2
Hexagonal 0.142
NbS
2
Hexagonal 0.098
TaS
2
Hexagonal 0.033
Diselenides MoSe
2
Hexagonal 0.178
WSe
2
Hexagonal 0.13
NbSe
2
Hexagonal 0.12
TaSe
2
Hexagonal 0.084
Ditellurides MoTe
2
Hexagonal 0.20
WTe
2
Orthorhombic 0.38
NbTe
2
Trigonal 0.70
TaTe
2
Trigonal 0.53
Graphite C Hexagonal 0.14
222
Tribology for Engineers
determined by the shear strength τ of the interfacial medium:
[5.55]
where A is the contact area over which shear force F is acting.
As discussed earlier in regard to friction modelling, it has
been found by Bridgman (1935) that
τ
is a function of the
contact pressure p. Thus,
[5.56]
and the pressure coeffi cient α determines the change in the
saturation shear stress with pressure. Bednar et al. (1993)
have re-examined the pressure dependence of the yield
strength and, using anvil experiments, determined the
effective friction coeffi cient μ
eff
of metals as a function of
applied pressure p and the saturation yield stress (represented
as τ in eq. [5.55]. Thus,
[5.57]
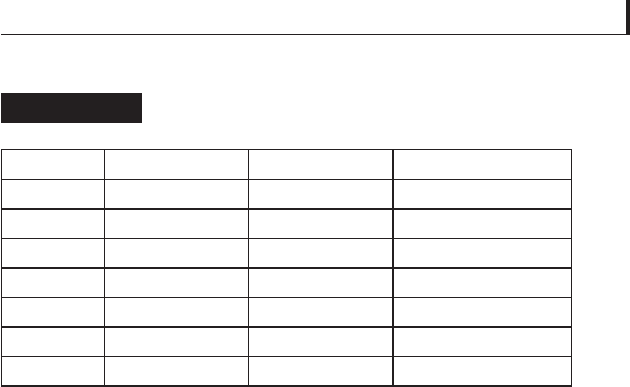
Experimentally determined values for
τ
o
and
α
of several
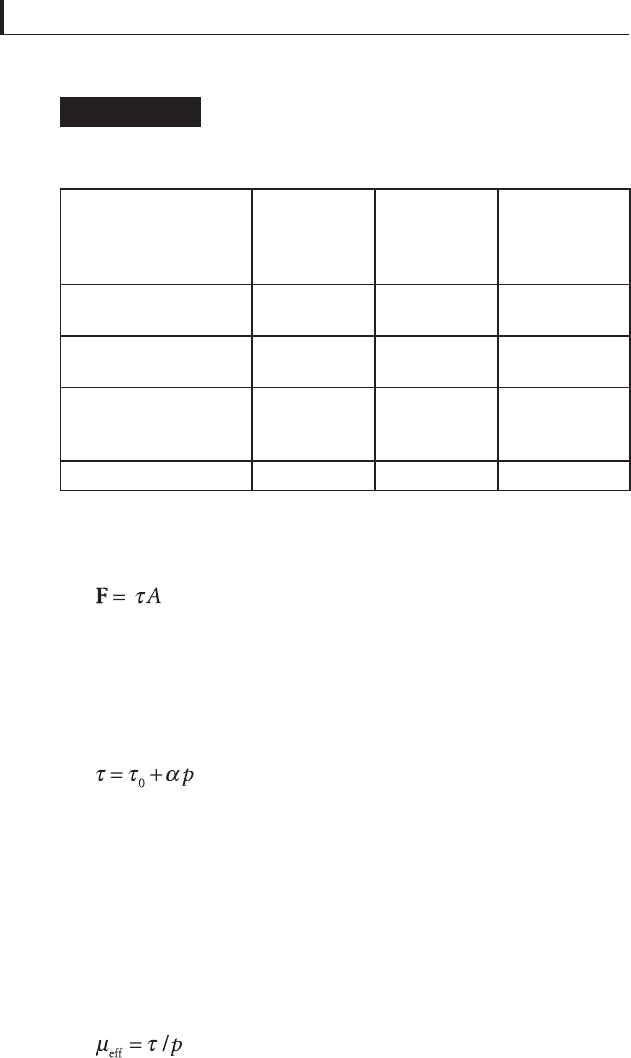
Effects of moisture on the friction coeffi cients
of various solid lubricants in air of various
relative humidity
Table 5.19
Solid lubricant
μ
k
, dry air, RH
< 6%
μ
k
, moist air,
RH = 85%
(after sliding
in dry air)
μ
k
, dry air
(after sliding
in moist air)
Molybdenum disulfi de
powder
0.06 0.20 0.06
Molybdenum disulfi de
bonded fi lm on disk
0.09 0.22 0.09
Molybdenum disulfi de
bonded fi lm on both
slider and disk
0.26 0.34 0.31
Graphite powder 0.06–0.10
a
0.16 0.19
a
Initial value before fi lm failure.
223
Tribology in manufacturing
metals are listed in Table 5.20. Of the three metals listed as
solid lubricants, silver and tin are used more than indium.
Interestingly, the frictional response of silver is quite suitable
for solid lubrication because there is no signifi cant difference
between μ
s
and μ
k
, leading to very smooth sliding with an
absence of stick-slip behaviour.
Graphite and molybdenum disulfi de are among the most
commonly used solid lubricants, and it is worthwhile to
consider their frictional behaviour specifi cally. Winer (1967)
compiled an extensive review of molybdenum disulfi de as a
solid lubricant that was published in 1967, and Fleischauer
and Bauer (1987) reviewed the chemistry and structure of
sputtered MoS
2
fi lms. Clauss (1972) has reviewed lubrication
by graphite. Both these materials are anisotropic in properties
due to their hexagonal crystal structures, which contain wide
separations between the basal planes. When properly
oriented, for example, by running in the surface to produce
easy-shear platelet orientations, friction coeffi cients for
MoS
2
can be as low as 0.02–0.1. Graphite typically exhibits
friction coeffi cients of 0.10–0.15 in air. Fluorination of
graphite to produce substoichiometric graphite fl uoride
Dependence of saturation shear strength and
friction of metals on the applied pressure
Table 5.20
Metal
␣
o
(MPa)
μ
eff
(at p = 1 MPa)
Fe 0.075 173.82 0.246
Cu 0.049 107.61 0.160
Ag
a
0.036 109.04 0.144
Au 0.029 91.82 0.123
AI 0.035 47.56 0.082
Sn
a
0.012 12.30 0.024
In
a
0.006 5.65 0.011
a
Commonly used solid lubricants.

224
Tribology for Engineers
CF
x
(0.3 ≤ x ≤ 1.1) increases the spacing between the basal
planes, making it a good candidate for future lubricants.
Another method to spread the basal planes and enhance
graphite lubrication is by intercalation. Intercalation involves
the insertion of atoms between basal planes. This process
can result in signifi cant enhancements of fi lm life as well as
frictional performance.
As discussed by Peace (1967), the maximum use temperatures
for graphite and molybdenum disulfi de depend on other factors
than temperature alone. These include relative humidity,
oxygen concentration in the environment, and whether the
material is in powdered or monolithic form. In furnace
oxidation experiments, graphite powder begins to oxidize
signifi cantly at about 585°C, compared with 298°C for
molybdenum disulfi de powder. Fusaro (1978) found that
oxidation causes molybdenum disulfi de fi lms to blister and
fail. This is explained by the tendency of molybdenum disulfi de
to form oxides and sulfi des of various stoichiometry in air. In
the case of graphite, the oxidation is highly anisotropic, but the
rates of oxidation are slower than for molybdenum disulfi de.
Bisson and Anderson (1964) prepared an extensive review
of solid lubricant properties, including graphite, molybdenum
disulfi de, and molybdenum trioxide. For example, the
friction coeffi cients of various MoS
2
and MoO
3
fi lms on steel
surfaces reaction to increasing sliding velocities is stunning.
Clearly, MoO
3
is a very poor lubricant. As the temperature
increases in air, molybdenum disulfi de undergoes changes in
colour and rate of oxidation. Table 5.21 summarizes these
changes, as discussed by Bisson and Anderson. Molybdenum
disulfi de and graphite can each be used as solid lubricants,
but attempts have been made to determine whether mixing
them together would provide synergistic effects. Gardos
(1987), for example, reviewed the use of graphite as an
oxygen scavenger to help molybdenum disulfi de fi lms retain
225
Tribology in manufacturing
low friction characteristics. Some limited advantages in
enhancing the stability and wear resistance of the
microcrystalline molybdenum disulfi de fi lms in air were
reported. About the same time, Bartz et al. (1986) in Germany
studied the friction of bonded fi lms containing various
combinations of graphite, molybdenum disulfi de, and
antimony thioantimonate [Sb(SbS
4
)]. Using a block-on-ring
apparatus, after sandblasting the 100CrMn6 steel ring, they
applied bonded fi lms to it, but left the 90MnCrV8 steel block
untreated. A method of assessing the effectiveness of the
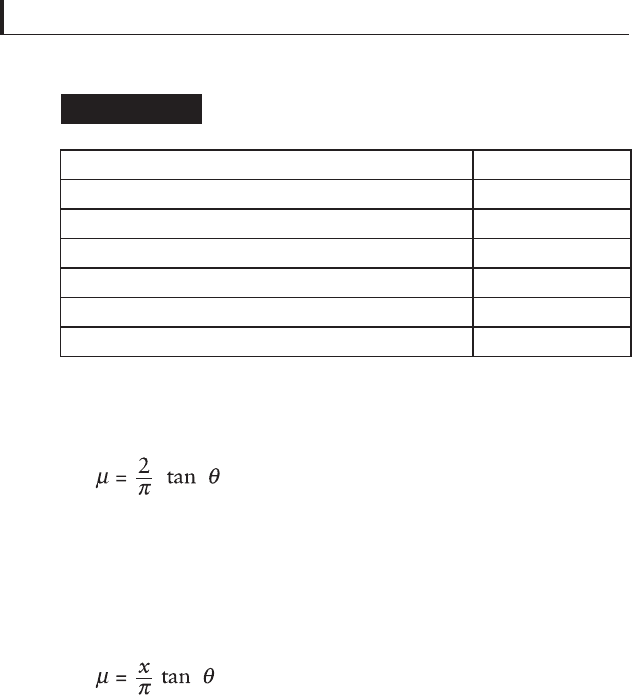
blends was to measure the stable, post-running-in friction
coeffi cient. Table 5.22 lists values of μ for several combinations
of lubricants.
Briscoe (1992) has reviewed the mechanisms of organic
polymer friction, stating that two non-interacting contributions,
adhesion and plowing, can be used to model behaviour. In
this treatment, frictional energy is dissipated by an interface
zone (adhesive) and a subsurface zone (deformation and/or
plowing). In the latter zone, behaviour in polymers may
be viscoelastic, plastic, or brittle. The friction coeffi cient
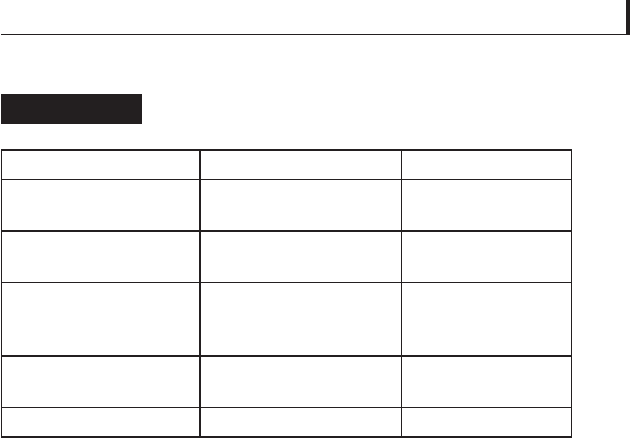
Transformations in molybdenum disulfi de as
temperature rises
Table 5.21
Temperature range (ºF) Temperature range (ºC) Behaviour
Up to 750 Up to 400 No detectable
oxidation rate
750 – 800 400 – 427 Thin oxide fi lm
forms
800 – 850 427 – 454 Slow, but
appreciable
oxidation
850 – 900 454 – 482 Yellowish white
MoO forms
Over 900 Over 482 Rapid oxidation
226
Tribology for Engineers
could be derived from geometric arguments to produce the
form
[5.58]
where the angle θ was associated with the roughness of the
surface. Briscoe found that if PTFE behaved in a more brittle
fashion, as it did after irradiation by gamma rays, the same
expression could be similarly written:
[5.59]
where the value of x, the slope of the dependence of friction
on tan
θ
, varied from 0 to 2 depending on the degree of
embrittlement (i.e., the extent of plastic fl ow). When tan θ
exceeded approximately 2.3, irrespective of x, the PTFE
began to exhibit chip-forming characteristics rather than
fl ow. Like other materials, the shear stress of PTFE was seen
to vary with contact pressure.
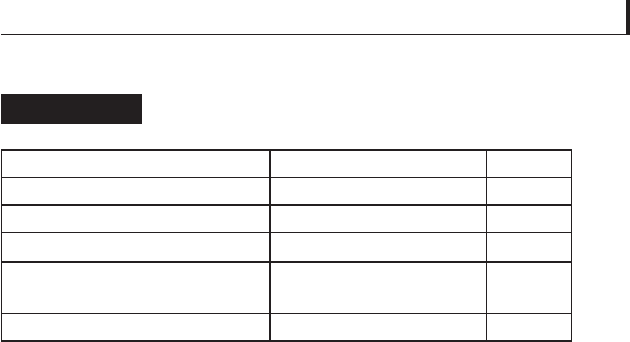
Since PTFE has a relatively low hardness, various additives
are mixed with it to improve its wear resistance. Table 5.23
shows the effects of certain additives on the friction coeffi cient
and wear of PTFE sliding on steel at 0.01 m/sec. Comparing
the fi rst and last row of data shows how it is possible to increase
wear resistance by more than three orders of magnitude while
Steady-state friction coeffi cients for solid
lubricant combinations
Table 5.22
Film composition
μ
, Steady state
Graphite alone Unstable
μ
MoS
2
alone 0.05
Graphite + MoS
2
(about 1:2 wt% ratio) 0.01 – 0.02
Graphite + Sb(SbS
4
) (about 3:4 wt% ratio) Unstable
μ
MoS
2
+ Sb(SbS
4
) (about 4:5:1 wt% ratio) 0.1 – 0.03
Graphite + MoS
2
+ Sb(SbS
4
) 0.04 – 0.05
227
Tribology in manufacturing
raising the sliding friction coeffi cient of the material by at most
about 0.03. Erdemir (1994) reviewed some of the important
mechanisms responsible for the lubricating action of solid fi lms
on ceramics such as silicon carbide, silicon nitride, and
aluminium oxide. He stated that solid lubrication may be the
only option available to help lubricate ceramics in severe
environments, but noted that like other types of lubricants,
solid lubricants suffer from fi nite lifetimes. He discussed the
use of boric oxide (B
2
O
3
) and its product with water, and boric
acid (H
2
BO
3
) in particular. Boric acid resembles other lubricants
with layered structures, and produces favourable friction
reductions under some circumstances. However, when the
temperature rises above about 170°C, boric acid decomposes
to boric oxide and loses its layered structure.
5.4 Tribology of rolling
Rolling is a process that cannot be conducted without friction
as friction is needed to draw the work piece into the roll gap
and to deform it. The minimum value of friction is twice that
needed for continuous rolling. The effects of friction are
connected to the geometric description of the process known
as the L /h ratio, where L is the projected length of the arc of
Effect of additives on the friction of blended
PTFE
Table 5.23
Material composition Wear rate improvement
a
μ
k
Unfi lled PTFE 1 0.10
15 wt% graphite 588 0.12
15 wt% glass fi bre 2857 0.09
12.5 wt% glass fi bre and 12.5
wt% MoS
2
3333 0.09
55 wt% bronze and 5 wt% MoS
2
4000 0.13
a
Ratio of the wear rate of unfi lled PTFE to that of the given material.
228
Tribology for Engineers
contact and h is the mean strip thickness. At L/h > 2, deformation
is homogeneous and the limiting strip thickness may be
reached. At L/h < 2, there is an inhomogeneity and at L/h < 1,
sticking friction occurs, so lubricants are applied to reduce
friction and wear. In cold rolling, lubricants are used to reduce
friction, although a minimum amount is required. Surface
fi nish requirements are friction dominated. In hot rolling,
lubricants are used to control adhesion between material and
roll. Lubricants may be oil or water based, and extreme
pressure additives are used where there is a mixed-fi lm
lubricating mechanism. The most commonly used lubricants in
rolling are shown in Table 5.24.
Commonly used lubricants and typical
μ
(friction coeffi cient) values in cold and hot
rolling
Table 5.24
Material Hot rolling
– lubricant
Hot rolling
–
μ
Cold rolling –
lubricant
Cold rolling
–
μ
Steel Water
Emulsion of
fat + EP
additive
Fat (ester) +
EP additive
+ water
Sticking
0.4
0.3
3–6% emulsion
of palm oil
0.01 – 0.03
Al and Mg
alloys
Emulsion,
2–15% of
mineral oil
0.4 Mineral oil with
1–5% fatty
acid
0.01 – 0.03
Cu and Cu
alloys
Emulsion,
2–8% of
mineral oil
0.3 2–10%
concentration
of mineral oil
with fat
0.01 – 0.03
Ti alloys Fat + water Sticking Esters or soap
Castor oil
Compounded
mineral oil
0.2
0.2
0.2
Refractory
metals
Dry 0.3 Mineral oil with
boundary and
EP additives
0.01–0.03
