Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.


684 33. MISCELLANEOUS TOPICS
9.2 to 9.8 is ideal. In low—pressure steam or hot
water heating systems, nitrite or chromate provides
a protective film on metal surfaces.
Filming amines provide a physical barrier to
protect metal from corrosion. They are effective
in the pH range of 5.5 to 8.5. Amines that are
conmionly used include those with long—chain
hydrocarbons that orient away from the metal
toward the water, such as octadecylamine.
Cy clohexylamine, diethy laminoethanol,
dimethylpropanolamine, and morpholine are
sufficiently volatile to travel with the steam. The
amine in the vapor helps keeps the pH sufficiently
high to avoid corrosion.
Scale control
Scale tends to build up in boiler water (called
water side deposits) for two reasons. First,
bicarbonate ions are converted to carbonate ions
and carbonate salts of calcium and some other
cations have low solubility. Second, the solubility
of calcium carbonate and similar compounds de-
creases with increasing temperature. Heat transfer
surfaces, where the temperature change is most
dramatic and fast, tend to be where scale forms.
The buildup of scale is controlled by the use
of dispersants with chelants and/or phosphate.
Chelants like EDTA (Section 14.7) keep cations
like calcium from precipitating by forming strong,
soluble complexes with them. Phosphate tends to
precipitate these materials early on so they do not
later precipitate on heat transfer surfaces. Com-
pare the solubility products of the compounds in
Table 13-5 to those of CaHP04, 1 x 10"^;
Ca3(P04)2, 2 X 10-2^; Ba3(P04)2, 3.4 x
lO'^^;
Mg3(P04)2, « 10-23 to 10-2^; and Zn3(P04)2, 9 x
10"^^
Remember, however, that the phosphate
salts of divalent cations have five species in the
solubility product.
EXAMPLE 1. Compare the concentration of
calcium ion in calcium carbonate and calcium
phosphate at
25
°C.
SOLUTION:
[Ca2^][C03-] = 4 X 10-^
[Ca2+] = 6.3 X 10-^
[Ca2+]3 [(P04^)]' = 2 X 10-2^
[Ca^-'] = (2
X
\0-^y^ = 1.8
X
10-"
This applies only at high pH (11—12) where
carbonate and phosphate ions occur. At
moderate pH (5—7) calcium bicarbonate is
fairly soluble (apparently too soluble to find
^sp values readily in tables), but calcium
hydrogen phosphate is still fairly insoluble.
PROBLEM: What is the solubility of calcium
over calcium sulfate and calcium hydrogen
phosphate? This is applicable at pH values
found in boiler water.
NTA (nitrilo triacetic acid) is a weaker
chelating agent than EDTA but is used for boiler
water treatment.
Excessive boiler water carryover with the
steam will cause corrosion and scale formation on
turbines and other critical equipment.
Demands of
high—temperature
boilers
New boilers for the pulp and paper industry
operate at 900—1500 psig. While water hardness
is always of concern, the formation of metal oxide
scales is equally important. Iron (and possibly
copper) oxide is often the principal contaminant of
scale formation in high—temperature boilers.
Polymeric dispersants have been used for
scale control at lower temperatures since the
1950s. These include negatively charged poly-
mers,
such as polyacrylate, polymethacrylate, and
polyacrylate—acrylamide copolymer, apparently
bind to the positively charged metal oxide
particles, keep them in solution, and prevent them
from growing, i.e., steric stabilization. (One
might hypothesize that maleic acid copolymers
may chelate better than these other polymers.)
Unlike chelants or precipitants, dispersants do not
react stoichiometrically.
In the mid—1980s Betz introduced a polyeth-
ylene polymer (with every other carbon containing
a methyl group and a phosphonate group) for use
at high temperature.
Boiler water
blowdown
As steam is removed from the boiler, salts
and other materials remain behind, allowing these
materials to concentrate in the boiler water. The
concentration of salts and metal oxides are typical-
ly 10—50 times higher in the boiler water than in
the feedwater. Some boiler water is continuously

BOILER FEEDWATER TREATMENT 685
removed from the system to prevent undue con-
centration of these materials that would eventually
precipitate and cause scale.
33.6 CORROSION
Corrosion
Corrosion, while always an important consid-
eration, has become of particular concern to the
pulp and paper industry since the 1970s, when
mills have been forced to reuse water in various
processes. As pressure continues to increase
water closure in mills, the problems of corrosion
will increase. Indeed, corrosion may be one of
the limiting factor in determining how much
closure can be obtained. Some basic aspects of
corrosion have been considered in Section 13.11
on page 330.
Types of metals for corrosion resistance
Table 13-8 (page 331) shows some metals
with their relative resistance to corrosion in mov-
ing sea water. A few additional aspects of stain-
less steels will be considered here. In addition to
the compositions shown in Table 13-8, most stain-
less steels have 1—2% Mn, although 201 and 202
have 5.5—7.5% and 7.5—10%, respectively. P is
limited to less than 0.06 or 0.045% in most
stainless steels, although type 303 allows up to
0.2%. S is limited to 0.15%, 0.06%, or 0.03% in
many types. Si is limited to 1.0% in many stain-
less steels; however, type 302B specifies
2—3%,
type 314 specifies 1.5—3%, and type 403 limits it
to 0.5%. Stainless steels with an L suffix are low
carbon. For example, types 304 and 316 have
maximums of
0.08%
carbon while 304L and 316L
have maximums of 0.03% carbon.
Type 317 is designed for maximum corrosion
resistance of standard stainless steels; its composi-
tion is 18—20% Cr, 11—15% Ni, 2% Mn, and
3-4% Mo. Type 347 is 17-19% Cr, 9-13%
Ni,
2% Mn, 1% Si, 0.045% P, 0.03% S, and at
least 10 times the amount of carbon as Cb—Ta.
In addition to the stainless steels, which have
matrixes of iron, alloys which are mixtures of
chromium and molybdenum in a nickel matrix are
very important.
Duplex stainless steels have been used in
selected corrosive environments since the 1960s.
New duplex stainless steels that are more easily
welded have been available since the mid—1980s.
These materials offer the potential to replace other
stainless steels in a wide range of applications.
Acid
cleaning
and
corrosion
(Crowe, 1992)
Many components, such as kraft digesters,
are washed with acid to remove scale. CaCOj and
other carbonates are major species of scale; acid
solubilizes this material by converting the COj^'
into CO2 and HjO by the addition of 2H+. When
HCl is used as the source of acid the remaining
CaCl2 is water soluble. Formic (HCOOH),
sulfamic, and nitric acids are also used for acid
washing. The use of additives (corrosion inhibi-
tors) with these acids may decrease the amount of
corrosion caused by acid washing. These inhibi-
tors are often organic sulfides or amines. One
inhibitor is /?-aminophenol, which has trade
names of Activol and Rodinal. Another agent is
diethylhydroxylamine and is commonly used in
steam cycles. Propargyl alcohol (2—propyn—1—
ol) is used in strong acid baths to decrease hydro-
gen embrittlement and steel corrosion. [The
addition of copper sulfate to hot, dilute, sulfuric
acid often limits its corrosion to stainless steel.]
Often paper mill workers allow suppliers to
give proprietary chemicals as corrosion inhibitors.
This gives the mill no way to effectively test it
against other manufacturers, products without
lengthy studies. Even research papers report
results with unknown inhibitors (Crowe, 1992). It
is also difficult to find information on corrosion
inhibitors that may be particularly effective to the
industry. A mill could even do its own corrosion
tests with small metal coupons in various solutions
to determine what might be most suitable (Crowe,
1992).
The coupon test is appropriate since acid
washing is a short—term corrosion.
Silicates are often other major components of
scale. But, as indicated on page 637, silicates are
soluble under highly alkaline conditions; there-
fore,
they are not present in many components of
the kraft process such as digesters, liquor evapora-
tors,
etc. On the other hand, aluminum, calcium,
and silicate together can form a troublesome scale.
The removal of scale by acid washing is a
tradeoff of many factors. Some acid washing
solution circulation is needed to help dissolve the
scale, but too much circulation leads to increased
rates of corrosion. The time of exposure to the

686 33. MISCELLANEOUS TOPICS
acid cleaning solution must be such that most of
the scale is removed, but not so long that the
amount of corrosion is unnecessarily high.
Ferric ion and iron sulfide in HCl increases
corrosion because as the ferric ion is reduced iron
metal is oxidized. Iron oxides are removed by
HCl treatment.
Nitric acid is often used instead of HCl for
cleaning stainless steel surfaces of digesters be-
cause HCl causes pitting or stress corrosion.
Nitric acid rapidly attacks carbon steel.
Formic acid vapors cause corrosion since the
inhibitors are not necessarily
volatile.
Formic acid
can decompose to CO and H2O, so it must be used
with ventilation and containers with relief valves.
Sulfamic acid (NH2SO3H) is used below
50°—60°C since higher temperatures (above about
70°C) leads to some formation of sulfate, which
causes scale formation by formation of insoluble
metal sulfates (such as CaS04).
Corrosion in the wash and
bleach
plants
As late as 1970, most brown stock pulp
washers were constructed of mild carbon steel, but
now stainless steel is specified.
Chlorine dioxide is surprisingly harsh on
nickel—based alloys. Even type 317L stainless
steel (3—4% Mo) is now considered inadequate
for many bleach plants. The stainless steels with
high levels (as much as 6%) of molybdenum are
fairly resistant to attack by CIO2 and have been
incorporated into many bleach plant washers.
Titanium apparently reacts very quickly with
dry chlorine so its use must be limited to areas
where exposure to dry chlorine is avoided. How-
ever, it seems to have very good corrosion resis-
tance in most bleach plants.
Corrosion in the recovery boiler
Corrosion in the recovery boiler is the most
difficult challenge to the pulp and paper mill
corrosion engineer. The potential for explosions
makes corrosion monitoring of the recovery boiler
very important. High temperatures, molten salts,
water, oxygen, and condensation ofmaterials all
contribute to the possibility of rapid corrosion
rates.
This area is discussed in detail by Sharp
(1992).
Prevention
of
corrosion in
boilers during shutdown
When power boilers are shut down water
may condense on the fireside surfaces if the
temperature of these surfaces falls below the dew
point. Sulfiir deposits may react with this water to
form very acidic solutions that can quickly corrode
the tubes. The corrosion is often localized, caus-
ing severe pitting.
This type of damage can
be
minimized during
shutdowns by taking the following steps (in the
order presented): removing deposits as quickly as
possible, washing the deposit—free surfaces with
water, air drying the surfaces, applying a thin
layer of lightweight oil, and using a desiccant,
such as unslaked lime, which is replaced as neces-
sary, in the ashpits.
To prevent problems in the future, one might
take several samples of deposits, dissolve them
separately in small amounts of water, and measure
the pH of the water. A pH below 4 may signal
potential problems with the sulfur content of the
fuel supply.
Corrosion
in
the
paper machine
Corrosion of bronze rolls on paper machines
was studied by Magar (1992). Five corrosion
inhibitors were tested that all contained mercapto-
benzothiazole as the active ingredient. The inhibi-
tors were all more effective if added prior to
exposure of the test liquid to the bronze.
Stress corrosion crack detection
The detection of cracks caused by stress
corrosion is often accomplished nondestructively
by the use of liquid penetrant or magnetic particle
tests.
One study (Reid and Reid, 1993) indicates
that some surface metal must be removed by light
surface grinding for these methods to be effective;
sandblasting and power wire brushing are inade-
quate preparation methods. The paper refutes the
idea that such grinding forms cracks or enlarges
existing cracks for commonly used alloys.
33.7 SAFETY PRACTICES FOR
HAZARDOUS CHEMICALS
Table 33-5 summarizes some steps to take
when handling potentially hazardous chemicals.
Material Safety Data Sheets are available from the
manufacturers and suppliers of chemicals.

WATER CONDITIONING 687
Table 33-5. Procedures for handling hazardous
chemicals.
1.
Read the Material Safety Data Sheet
(MSDS).
2.
Follow the manufacturer's directions for han-
dling the material.
3.
Label all vessels and plumping carrying the
material in a clear fashion with indelible ink.
4.
Be sure to use appropriate protective equip-
ment, clothing, gloves, and safety glasses or
face shields. Have plenty of these materials
on hand, near the point of use, so they are
used.
5.
Do not handle these materials or feed them
into the process by hand; instead use high-
quality metering pumps equipped with power
interlocks to prevent their addition during
shutdowns.
6. Locate showers and eye wash stations near
the point of use of toxic chemicals.
7.
Do not smoke or eat when handling these
materials.
8. Do not wipe or scratch your eyes, mouth,
head, or other body parts with your gloved
hands or clothing that has been in contact
with poisonous or corrosive chemicals.
33.8 TRANSPORTATION SAFETY
Introduction
This section was prepared with the help of
Mankui (David) Chen who collected most of the
references and wrote some of the first draft of the
text. The author grateftiUy acknowledges his help.
The chemical industry occupies an important
place in the U.S. economy; its total output ac-
counts for about 10% of the GNP in manufactur-
ing. Hauling chemicals is a major source of
revenue for railroads and motor carriers. The
share of freight handled by rail has decreased from
about 70% in 1946 to less than 40% in 1990.
Trucking's share increased from 5 to 25% in the
same time. The railroad industry was deregulated
with the Staggers Rail Act in 1980. In 1989 there
was a record year of nearly 1.5 million rail car-
loads of chemicals and related materials.
Intermodal transportation involves shipping a
material with several forms of transportation (a
combination of truck, ship, rail, and even air).
For example, a motor carrier might deliver and
pick up a bulk handling container at a plant. The
container would be transported by rail over a large
distance and then transferred to a motor carrier for
transportation to the final destination. This trend
has not been widely practiced in the past since the
railroad and trucking industries have been major
competitors rather than cooperators. The ability
of a shipper to deal with one carrier is a driving
force for the partnerships that are forming between
the various types of carriers.
Chemicals can be classified into hazardous
materials and nonhazardous materials. Hazardous
materials may be corrosive, explosive, flanmiable,
and/or toxic. Any major accident in their trans-
portation can directly endanger people's lives and
the environment. Hazardous material handling
regulations include substance classification (corro-
sive,
explosive, etc.), packaging, handling, label-
ing and placarding, accident reports, information
hotlines, filing route plans, etc.
Some incidents involving "nonhazardous"
chemicals may not threaten people, but can de-
stroy an entire ecosystem. For example (Elmer—
DeWitt, 1991), a Southern Pacific tanker car
derailed on a treacherous canyon bridge six miles
north of Dunsumuir on July 14, 1991. Its contents
of 19,500 gal. of metam sodium spilled into the
Sacramento River. Metam sodium was classified
as a nonhazardous material at the time of the
accident. It wiped out aquatic plants, nymphs,
caddis flies, mayflies, and at least 100,000 trout
through a 45 mile stretch.
Every year there are about four billion tons
of hazardous materials and radioactive waste
shipped in the U.S. Accidental chemical releases
seem inevitable—every day there are about 20
accidents where hazardous materials are released
in interstate transportation. These accidents result
in hundreds of deaths, thousands of injuries, great
loss of resources, and damage to the environment.
The public grows increasingly concerned about
this environmental issue.

688 33. MISCELLANEOUS TOPICS
Government agencies and Congress react to
this situation by proposing new laws. Chemical
companies are taking many steps to ensure the safe
handling of their products as the liability is shifting
from the carrier to the shipper.
Safety regulations and codes
There are many regulations, rules, and codes
concerning the transportation of hazardous materi-
als issued by the federal government, the states,
associations, and manufacturers. Three very
important and influential regulations from the
federal government, the shipper, and the carrier,
respectively, are considered here as examples.
The Hazardous Materials
Transportation
Act
of 1974 was the first complete federal law about
hazardous materials transportation; it was origi-
nally passed by Congress as the result (in part) of
public concern about the air accident at Logan
Airport in 1974 (Gerhart, 1992). Three crewmen
aboard a Boeing 707 died in a crash at Logan
Airport in Boston, Mass., in 1974. Investigators
found the dead men had been incapacitated by
ftimes from 10 quarts of improperly packaged
nitric acid. Although correctly marked and la-
beled, the acid was stored on its side and leaked.
The regulation covers substance classification,
packaging, handling, incident report, placarding,
and other topics.
In November 1990, Congress approved the
Uniform Safety Amendments for this act (Stitt,
1991).
It introduced a long list of new regulations
covering emergency response information systems,
specific highway routes, training programs, regis-
tration requirements, safe permit system, inspec-
tion and certification, and in place financial re-
sponsibility (insurance).
This bill allows federal preemption of state
and local hazardous materials laws, calls for state
and local participation in setting routes and stan-
dards,
and sets up a petition process for objections
to routing of hazardous materials. It expanded
federal authority to prosecute violators and in-
creased civil and criminal penalties.
Responsible Care (McConville, 1992;
Ainsworth, 1993) is considered the standard for
the chemical makers' total approach to carrying
out business. It was set up in 1988 by the Chemi-
cal Manufacturers Association (CM
A),
launched in
1989,
and parts of it continue to be ratified. It
provides a framework for good safety practice to
guide manufacturers, distributors, and carriers. It
consists of six operating codes covering every
facet of the industry:
•Community awareness and responsible care
•Pollution prevention
•Process safety
•Distribution
•Employee health and safety
•Product stewardship
Product stewardship is a catch—all for mak-
ing "health, safety, and environmental protection
an integral part of designing, manufacturing,
marketing, distributing, using, recycling, and
disposing of products."
The most stringent code for logistics is the
Distribution Code of Management Practice, which
includes the following five elements:
A. Risk management
B.
Compliance review and training
C. Carrier safety
D.
Handling and storage
E. Emergency procedures
The purpose of this code is to reduce the risk
of chemical exposure to the public, carriers,
distributors, chemical industry employees, and the
environment. To meet the requirements of this
code,
CMA's 178 member companies had to
make drastic changes to their policies and proce-
dures and document their compliance.
Many of the goals of the program are bor-
rowed from TQM such as "constantly upgrading
manufacturing procedures and practices."
The Key Train and Key Route Program is a
program from chemical carriers designed to
improve safety and assure compliance with regula-
tions.
One of the most advanced cross—industry
efforts has brought together representatives of the
railroad, chemical, and rail equipment industries to
develop standards to improve safety. The group,
the Inter—Industry Task Force on the Safe Trans-
portation of Hazardous Materials by Rail, called
for defining trains and routes of highest concern as
"key trains " and "key routes" for limiting train
speeds (50 mph) and for enhancing track mainte-
nance and derailment safeguards. The regulation

TRANSPORTATION SAFETY 689
detailed practices for yard operations, storage, and
employee training (Anon., 1990).
In addition to the regulations listed above,
there is a new suggestion that carriers obtain ISO
9000 certification to compete in the international
market. TRANSCARE (The Transportation
Community Awareness and Emergency program)
calls for the combination of community education
and emergency response training with public
relation efforts.
Carriers
According to the Interstate Commerce Act,
54,
Statute 889, carriers are classified as public or
private. Private carriers are carriers that are (an
incidental) part of the shipping company through
ownership and/or leasing; they are not subject to
government price regulations.
Public carriers can be common, contract, or
exempt. Common carriers agree to serve all who
wish to do business, and their prices are regulated
by the government. Contract carriers are bound
by a contract between the carrier and the shipper,
are limited in the number of firms they can serve,
and are not subject to government price control.
Exempt carriers are not subject to government
price control but are limited to certain products,
geographical regions, and organizations formed.
All carriers are subject to government safety
regulations.
To comply with government regulations and
meet public expectations, logistics departments of
chemical companies are putting a great deal of
effort in the selection of carriers, establishment of
partnerships with carriers, and improvement of
communication with carriers and communities.
Partnerships
Partnership relations between shippers and
carriers are now becoming standard for logistics
management (Rotman and Gibson, 1989; Morris,
1990).
Companies like Du Pont, ICI, and
Monsanto are giving this much concern. They
believe that if service can be improved and if
safety regulations can be completely carried out,
a carrier should be a partner from the beginning.
By reducing their carrier base and by formalizing
that relationship with contract carriers, many
shippers achieve high standards of safety and
performance.
Chemical shippers are reducing their carrier
bases to a few trusted companies; at the same
time,
they are attempting to establish more durable
relationships and monitor more closely the safety
and service of
the
selected carriers. For example,
Monsanto cut its carrier base from 1500 to 100
within three years; PMC's chemical products
group slashed its base of bulk carriers from 40 to
12 and packaged carriers from 500 to 60 in 1992.
With fewer carriers, the existing carriers obtain
more business and gain incentive to improve
service and safety.
One approach used by some companies to
ensure safety and service standards by truck
carriers involves the use of contract rather than
common carriers. For example, Du Pont uses
about 95% of its bulk truckers on a long—term
contract basis. As a major step in beginning its
comprehensive safety program. Ethyl Co. decided
that all shipments of hazardous materials would be
by contract carriers (Cooke, 1992) using Ethyl
owned or leased trailer and tank equipment. Dow
Chemical Co. contracts most of its truck transpor-
tation with motor carriers to ensure that they
adhere to its standards for safe handling. These
companies got away from the traditional, pre-
regulation relationship with carriers and built up
close relationships with contract carriers, sharing
information and even objectives, and in some case
contract carriers could paint their trucks to match
the companies' logos.
Selection of
carriers
and drivers
Carefully scrutinizing transportationproviders
is the first and critical step for hazardous material
shippers to achieve safe transportation. The
screening processes used by some companies gives
us a good model. Auditors from the Department
of Transportation (DOT) are responsible for
checking every aspect of a carrier's safety tech-
niques, from drivers' logs to equipment mainte-
nance records. If a DOT auditor finds violations,
he or she will rate the carrier accordingly with
three options: satisfactory, conditional, and unsat-
isfactory. Most companies require that the hazard-
ous material carriers have the satisfactory rating.
Some shippers also carry out on—site surveys of
all their contract carriers. Du Pont and Rohm and
Haas perform thorough evaluations and on—site
surveys. They go into their multiple facilities and

690 33. MISCELLANEOUS TOPICS
review maintenance programs, logs and training.
They want to see how safe the carriers are with
their products (Gordon, 1990).
Besides examining the DOT audit and
on—site survey, some shipping companies think it
is necessary to train the drivers themselves; Du
Pont trains carrier employees extensively. Its
hazard material training program called RHYTHM
(Remember How You Treat Hazardous Materials)
has become an industry standard (Gordon, 1990).
Ethyl Co. (Cooke, 1992) has developed its own
instructional and certification program for the
drivers of the contract motor carriers who have
excellent safety records at the time of their selec-
tion. Ethyl wants to be sure that the carriers'
drivers are fully knowledgeable about the chemi-
cals they would be hauling and to be sure that the
drivers are taught appropriate emergency—re-
sponse procedures including proper use of person-
al protective equipment. The principal benefit of
this program is the improved safety in transporting
their hazardous materials.
Chemical distribution
is
growing increasingly
sophisticated and requires improved communica-
tion.
Traditional paper documentation and the
hotline system called CEMTRCE (an emergency
response communication standard) seems unsuit-
able.
Electronic data interchange (EDI) into a
nationwide computer system
has
to be established.
The system would provide fire fighters with the
exact details on all U.S. shipments of hazardous
materials and provide emergency personnel with
dial—up access to a mainframe database of elec-
tronic manifests listing details regarding the con-
tent of every hazardous materials' shipment.
Since the present EDI technology is not advanced
enough to process this vital information, the
proposal to the Congress to mandate the use of a
national computer tracking system has not been
passed yet (Hamilton, 1993).
33.9 OPERATIONS MANAGEMENT
Operations are used to convert resources
(inputs include people, capital, raw materials) into
products (output). Products are usually either
manufactured goods (tangible products like a new
car) or services (intangible goods such as banking
services). The output should have more value
than the input to "create wealth."
o
Operations management is the decision-
making process whereby all aspects of the process
are selected based on the criteria selected for the
process. Processes must be flexible to respond to
changing market conditions and customer needs,
efficient in order to capture market share from
other competitors,
and
produce high—quality prod-
ucts.
Requirements for an operation include
research and development to design a product;
engineering to scale the process to industrial size;
capital for equipment, facilities, inventory, and
raw materials; marketing; accounting; human
resources; and information systems.
Other criteria include product safety and
limited environmental impact. Consumers and
society have placed higher values on these criteria
in recent years.
A system consists of a group of related
activities, items, and events. A change in one
item will cause a change in other part(s) of the
system. Any system is a part of a larger system
and is usually made up of subsystems.
Flow charts are used to show overall
processes. Process chart symbols include
^^.^^ an "o" for operation, arrow for
' 1^ transportation, triangle for storage, D for
n
delay, and square for inspection. Some-
times symbols are combined if operations
are combined; for example, a triangle
inside a square indicates storage and
inspection.
Flow charts are often used in con-
junction with time and motion studies to reduce
bottlenecks and improve the overall process.
33.10 PROCESS SIMULATION
Introduction
Process simulation in pulp and paper usually
implies the mathematical solution of mass and
energy balances facilitated by the use of comput-
ers.
[There are many other types of simulation as
well, such as simulating the steps in an industrial
process or a grocery store. In the latter, the
computer would "create" customers that enter the
store at times based on a probability distribution.
These customers would spend various lengths of
time in the store and purchase various items. The
average length of
time
(and variability) they would
be required to spend in a checkout line (queue)
D

PROCESS SIMULATION 691
and other parameters could be determined. These
simulations allow one to predict where bottlenecks
will occur and how scheduling of employees might
change the situation.]
Steady—state
simulators work on the assump-
tion that variables are constant with time or reach
a constant level. The heart of simulation is the
selection of mathematical equations that represent
the relationships between
variables.
These mathe-
matical equations are called models. In turn,
many of the mathematical equations are based on
certain assumptions.
The results of simulation are as good as the
assumptions of the models, the models, and the
degree of precision of the actual values of a
variable. The sensitivity of a variable is the
amount that a small change in the value of that
variable causes the final result to change. If a
small change in the value of a variable causes a
large change in the result, it is important to know
the value of that variable with a high level of
precision. The term garbage—in—garbage—out is
used to describe simulation processes where there
is a high level of uncertainty in assumptions,
models, and the actual values of variables, so the
results are essentially meaningless.
Simulation does not have to be elaborate to
be useful and effective. Some of the most useful
simulations are quite simple. The degree of cook
in the kraft process is modeled by the H—factor
which combines the variables time and tempera-
ture.
The mathematical relationship is sound and
the variables of time and temperature can both be
measured with a high degree of precision. The
effects of time and temperature of the pulping
process on the yield can be predicted accurately
for an infinite number of combinations ahead of
time.
That is why this has enjoyed widespread
use.
The model does not describe the influence of
pulping chemicals on the pulp yield but other
models with these refinements have been described
(page 371). Section 17.6 describes the simulation
of fiber cleaning systems.
Process simulation, however, usually implies
the use of a specially designed program to keep
track of large numbers of variables. The first
process simulation programs of large scope were
developed in the late 1960s. Several popular ones
(roughly in order of decreasing popularity) for
pulp and paper include GEMS, which was devel-
oped by L.L. Edwards at the University of Idaho,
MAPPS,
which was developed by the Institute of
Paper Chemistry and is a modification of GEMCS,
MASSBAL, developed by SACDA at the Univer-
sity of Western Ontario, and FlowCalc, which is
written in Basic and is designed specifically for
microcomputer use; all of
these
have versions that
run on IBM/PC compatible computers. Many
people are performing sophisticated simulations
using spreadsheet programs such as QuattroPro®
or Lotus
1—2—3®.
Models should
be
checked for agreement with
reality by
validation.
The model should be tested
under several sets of conditions (where the re-
al—world results are known) that were not used to
develop the model. If
the
model consistently gives
results that are in agreement with real—world re-
sults,
then it is probably very useful. However,
one should be careful to not used the model under
conditions where its underlying assumptions are
not valid or it has not been validated.
Once a process has been adequately modelled
and validated, variables can be changed in the
computer and the result will be determined. This
allows a large number of conditions to be studied
without having to perform numerous, expensive
mill trials. Processes can be made more efficient
or economically favorable. It may also be a useful
tool for operator training.
Simulation a particular process
As always, one must clearly define the
objectives of the simulation project. The simula-
tion of a process requires much background
information on the process. What variables are
involved? What are the relationships between the
variables? What equipment will be used in the
process? What are the typical operating condi-
tions?
This information is used to formulate
mathematical models. These models are then
converted into a form that the process simulation
program can use. The model is then tested and
validated. The model can then be used under to
predict real—world behavior.
Processes may be dynamic or steady—state.
The grocery store is an example of dynamic
simulation. Sometimes there may be many people
in the store, and other times there may be very

692 33. MISCELLANEOUS TOPICS
few. The values of variables of dynamic process-
es change with time. Most process simulation is
approximated by assuming the process reaches a
steady state where the values of the variables do
not change with time after steady state is reached;
in this case the system can be modeled as a series
of algebraic equations. The two principal solution
algorithms of steady state simulations are sequen-
tial and
simultaneous.
In sequential simulation the
variables are solved sequentially in a defined
order, solved again as better values for each
variables are determined, and resolved until the
convergence (change in the value of the variables
from a solution to the next solution) is within
acceptable limits. With simultaneous solutions
each equation is put into a linear form and all of
the equations are solved simultaneously.
33.11 SUPERABSORBENCY
Superabsorbent polymers (SAPs) have been
used in hygiene products since the late 1960s
(Masuda, 1993). About 250,000 tons are used
globally each year, most of which are used in
disposable diapers for babies. Most SAPs for hy-
giene products are the sodium salts of moderately
crosslinked polyacrylic acid (not all of the acid
groups are in the salt form) in a dry powder of
300 iim particles. The crosslinking agents are
typically glycol diacrylate or A^,Ar—methylene-
bisacrylamide. Residual monomer (which may be
a skin irritant) levels are now below 100 ppm.
The famous polymer scientist P. J. Flory had
theoretically determined the important parameters
for water absorption by polymers. The important
factors for absorption capacity include a polymer
with a high affinity for water (i.e., a polar poly-
mer),
a low crosslinking density in the polymer
(just enough to keep the polymer insoluble), and a
high ion density on the polymer.
Electrolytes dissolved in the water to be
absorbed decrease the amount of water ultimately
absorbed by the crosslinked polymer, and here the
type of electrolyte is important, with polyvalent
cations decreasing absorption to a higher degree
than monovalent cations (as expected from colloid
chemistry principles); these factors become
important when SAP systems are extended to non-
hygiene product uses such as those pressed be-
tween two papers and used in sheet form.
The rate of absorption is determined by
physical properties, especially the surface area of
the SAP. Finer particles generally absorb faster,
although if the particle size is too small, a gummy
material is obtained that does not absorb water
quickly. Masuda (1993) states that diaper dryness
is correlated to absorbency under load (AUL, r =
0.81) and diaper leakage is inversely correlated to
gel—stability (r = 0.93). The trend has been to
use lower amounts of fluff fiber and higher levels
of SAP in hygiene products; these products may
be as much as 30% SAP.
33.12 BEARINGS
Introduction
This section will provide a brief overview of
the different types of bearings and their use. This
information comes largely from the SKF USA Inc.
(King of Prussia, Pennsylvania) Product Service
Guide (April, 1992). Fig. 33-5 shows the basic
types of bearings that are discussed here.
Bearings range in size from the precision
miniature bearings used in medical and aerospace
applications to deep grove ball bearings with bore
sizes exceeding 55 in.
Single
row
deep groove
ballbearings—Conradtype
This popular type of bearing is a relatively
simple, nonseparable (since elastic deflection of
the rings is used to introduce the balls into the
bearing during the Conrad assembly method)
design. It can carry significant radial loads, and,
due to the uninterrupted raceway grooves and the
high degree of conformity between balls and
raceways, substantial thrust loads in either direc-
tion, even at very high speeds. Single row deep
groove ball bearings are available in open form or
with various types of seals or snap rings.
Single row deep groove ball bearings of the car-
tridge—type
Cartridge—type bearings have the same stan-
dard bore and outside diameter as single row deep
groove ball bearings but are as wide as double row
bearings. They are supplied with two seals or
shields.
Max type
(filling
slot)/single row ball bearings
Max type single row ball bearings (also
called filling slot type) employ a filling slot (rather
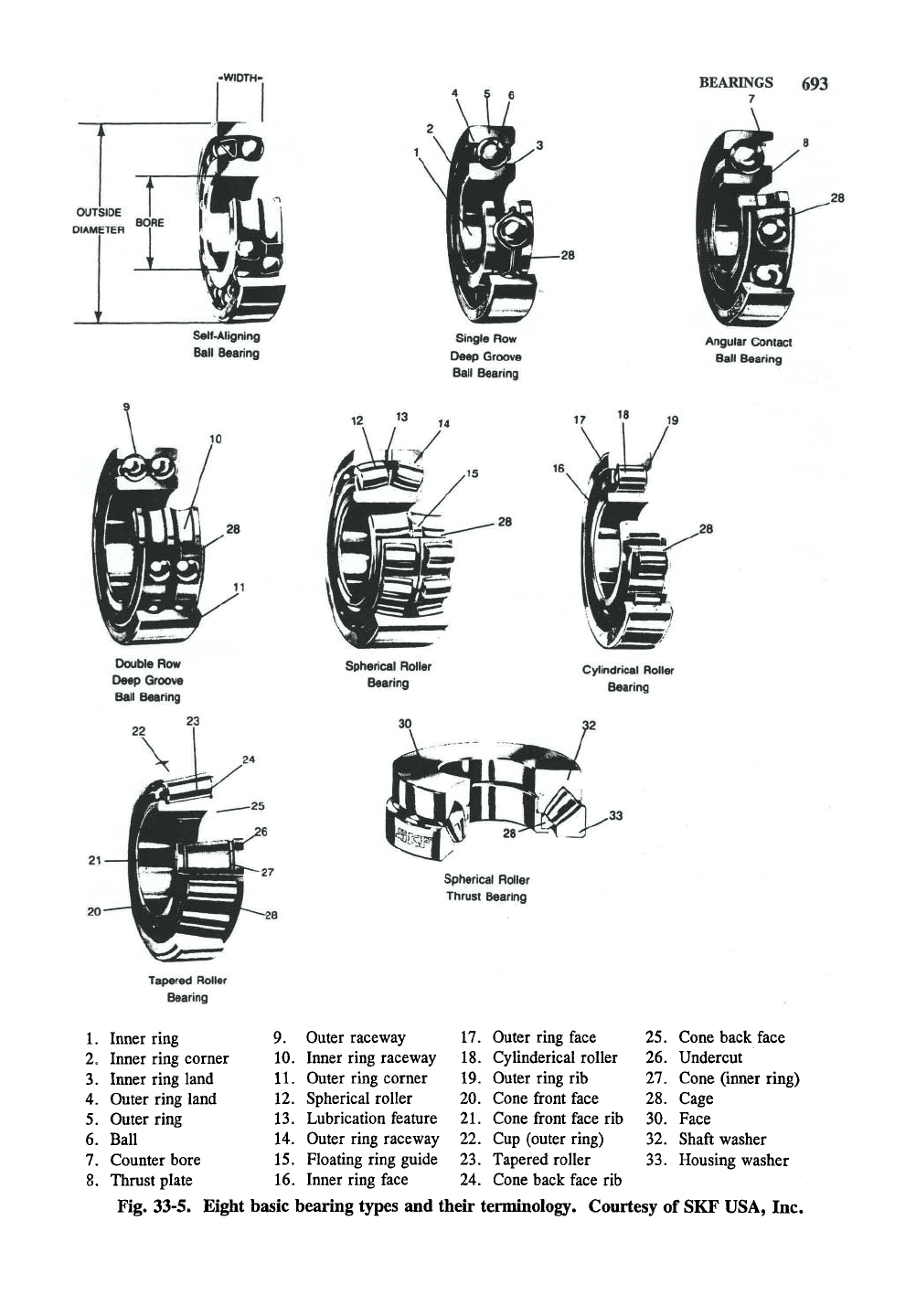
-WIDTH-
Self'Aligning
Ball Bearing
BEARINGS 693
7
Single Row
Deep Groove
Ball Bearing
Angular Contact
Ball Bearing
Double Row
Deep Groove
Ball Bearing
Spherical Roller
Bearing
Cylindrical Roller
Bearing
Spherical Roller
Thrust Bearing
Tapered Roller
Bearing
1.
2.
3.
4
Inner ring
Inner ring corner
Inner ring land
Outer ring land
5.
Outer ring
6. Ball
7.
Counter bore
8. Thrust plate
9.
10.
11.
12.
13.
14.
15.
16.
Outer raceway
Inner ring raceway
Outer ring corner
Spherical roller
Lubrication feature
Outer ring raceway
Floating ring guide
Inner ring face
17.
Outer ring face
18.
CyUnderical roller
19.
Outer ring rib
20.
Cone front face
21.
Cone front face rib
22.
Cup (outer ring)
23.
Tapered roller
24.
Cone back face rib
Fig. 33-5. Eight basic bearing types and their terminology. Courtesy of SKF USA, Inc
25.
26.
27.
28.
30.
32.
33.
Cone back face
Undercut
Cone (inner ring)
Cage
Face
Shaft washer
Housing washer
