Betsy T., Keogh J. Microbiology Demystified
Подождите немного. Документ загружается.


Fleming grew cultures of Staphylococcus aureus, a bacterium, in the laboratory.
He was also conducting experiments with Penicillium notatim, a mold. By acci-
dent the Staphylococcus aureus was contaminated with the Penicillium notatum,
causing the Staphylococcus to stop reproducing and die. Penicillium notatum be-
came one of the first antibiotics. An antibiotic is a substance that kills bacteria.
A summary of the scientists and their contributions can be found in Table 1-2.
CHAPTER 1 The World of the Microorganism
17
Fig. 1-9. Penicillium notatum is a fungus
that kills the staphylococcus aureus.
Table 1-2. Scientists and Their Contributions
Year Scientist Contribution
1590 Zacharias Janssen Developed the first compound microscope.
1590 Robert Hooke Observed nonliving plant tissue of a thin slice of
cork.
1668 Francesco Redi Discovered that microorganisms did not spontane-
ously appear. His contribution led to the finding that
killing the microorganism that caused the disease
could prevent the disease.
1673 Antoni van Invented the single-lens microscope, grinding the
Leeuwenhoek microscope lens to improve magnification. First
person to view a living organism.
1798 Edward Jenner Developed vaccinations against disease-causing
microorganisms.
c01_betsy.qxd 5/11/05 2:22 PM Page 17
CHAPTER 1 The World of the Microorganism
18
Year Scientist Contribution
1850s Mathias Schleiden, Developed cell theory.
Theodore Schwann,
Rudolf Virchow
1847 Ignaz Semmelweis Reported a dramatic decline in childbirth fever after
physicians used antiseptic techniques when deliver-
ing babies.
1864 Louis Pasteur Discovered that microorganisms were everywhere,
living on organisms and in nonliving things such as air.
His work led to improved sterilization techniques called
pasteurization. One of the founders of bacteriology.
1867 Joseph Lister Reduced infections after surgery by spraying car-
bolic acid over the patient before bandaging the
wound. This was the first surgical antiseptic.
1876 Robert Koch Discovered how microorganisms spread contagious
diseases by studying anthrax. Developed the
Germ Theory. Developed techniques for cultivating
microorganisms.
1870s John Tyndall, Discovered that some microorganisms are resistant to
Ferdinand Cohn certain sterilization techniques. One of the founders
of bacteriology.
1884 Elie Metchnikoff Discovered that white blood cells (leukocytes)
engulf and digest microorganisms that invade the
body. Coined the word phagocytes. Founded the
branch of science called immunology.
1887 Richard Petri Developed the technique of placing agar into a spe-
cially designed dish to grow microorganisms, which
was later called the Petri dish.
1890 Paul Ehrlich Developed the first drug to fight disease-causing
microorganisms that had already entered the body.
1928 Alexander Fleming Discovered Penicillium notatum, the fungus that
kills staphylococcus aureus, a microorganism that is
a leading cause of infection.
Table 1-2. Scientists and Their Contributions (Continued)
c01_betsy.qxd 5/11/05 2:22 PM Page 18

Quiz
1. What is a microorganism?
(a) A microorganism is a small organism that takes in and breaks down
food for energy and nutrients, excretes unused food as waste, and is
capable of reproduction.
(b) A microorganism is a small organism that causes diseases only in
plants.
(c) A microorganism is a small organism that causes diseases only in
animals.
(d) A microorganism is a term that refers to a cell.
2. What is a pathogenic microorganism?
(a) A microorganism that multiplies
(b) A microorganism that grows in a host
(c) A microorganism that is small
(d) A disease-causing microorganism
3. Name the parts of this microorganism using the nomenclature system:
Mycobacterium tuberculosis.
(a) A bacterium is a one-cell organism that does not have a distinct
nucleus.
(b) Mycobacterium is the presemous and tuberculosis is the specific
postsemous.
(c) Mycobacterium is the epithet and tuberculosis is the specific genus.
(d) Mycobacterium is the genus and tuberculosis is the specific epithet.
4. Why is a bacterium called a prokaryotic organism?
(a) A bacterium is a one-cell organism that does not have a distinct
nucleus.
(b) A bacterium is a one-cell organism that has a distinct nucleus.
(c) A bacterium is a multicell organism that does not have a distinct
nucleus.
(d) A bacterium is a multicell organism that has a distinct nucleus.
5. Why is a fungus called a eukaryotic microorganism?
(a) Fungus has cells that have a nucleus, nuclear envelope, cytoplasm,
and organelles.
CHAPTER 1 The World of the Microorganism
19
c01_betsy.qxd 5/11/05 2:22 PM Page 19

(b) Fungus has cells that have a nucleus and no nuclear envelope.
(c) Fungus has cells that have a nucleus, nuclear envelope, cytoplasm,
but no organelles.
(d) Fungus has cells that have no nucleus, no nuclear envelope, no cyto-
plasm, and no organelles.
6. What is Archaea?
(a) Archaea is a classification for organisms that have two nuclei.
(b) Archaea is a classification for organisms that use phagocytosis.
(c) Archaea is a classification of an organism that identifies prokaryotes
that do not have peptidoglycan cell walls.
(d) Archaea is a classification of an organism that identifies prokaryotes
that have peptidoglycan cell walls.
7. What is phagocytosis?
(a) The ability of a cell to reproduce.
(b) The ability of a cell to move throughout a microorganism.
(c) The ability of a cell to engulf and digest solid materials by use of
pseudopods, or “false feet.”
(d) The ability of a cell to change shape.
8. What is a compound microscope?
(a) A microscope that has one lenses.
(b) A microscope that has two sets of lenses: an ocular lens and an eye-
piece.
(c) A microscope whose lenses are concave.
(d) A microscope whose lenses are convex.
9. What is Germ Theory?
(a) Germ Theory states that a disease-causing microorganism should be
present in animals infected by the disease and not in healthy animals.
(b) Germ Theory states that a disease-causing microorganism should be
present in healthy animals and not in infected animals.
(c) Germ Theory states that a disease-causing microorganism should be
destroyed.
(d) Germ Theory states that a disease-causing microorganism cannot be
destroyed.
10. What is Edward Jenner’s contribution to microbiology?
(a) Edward Jenner discovered the Germ Theory.
CHAPTER 1 The World of the Microorganism
20
c01_betsy.qxd 5/11/05 2:22 PM Page 20

(b) Edward Jenner discovered how to create vaccinations to trigger
the body’s immune system to develop antibodies that fight micro-
organisms.
(c) Edward Jenner discovered the compound microscope.
(d) Edward Jenner discovered the compound nomenclature system.
CHAPTER 1 The World of the Microorganism
21
c01_betsy.qxd 5/11/05 2:22 PM Page 21
c01_betsy.qxd 5/11/05 2:22 PM Page 22
This page intentionally left blank.
2
CHAPTER
23
The Chemical
Elements of
Microorganisms
No doubt you’re asking yourself what chemistry has to do with microbiology
since they seem to be two different branches of science. The simple answer is
that microorganisms are made up of chemicals, as is every organism—and all
matter. Remember that matter is anything that occupies space and has mass.
You might say that an organism is a chemical processing plant where things
are broken down into chemical elements; these chemical elements are then re-
arranged to form new things. You do this every time you ingest food. Food is a
group of chemical compounds. The digestive process rearranges these digested
chemical compounds into new substances that provide you with energy and
nutrients that are necessary for you to live. Some microorganisms called auto-
trophic organisms manufacture their own food. Microorganisms that derive energy
from other microorganisms (food) are called heterotopic organisms.
c02_betsy.qxd 5/11/05 2:24 PM Page 23
Copyright © 2005 by The McGraw-Hill Companies, Inc. Click here for terms of use.

When you catch a cold or become infected by pathogenic microorganisms,
your body is no longer in homeostasis. You feel rotten, but what’s really hap-
pening is that the microorganism is disrupting your chemical processing plant’s
normal operation. Some microorganisms prevent necessary chemical processing
from occurring. Other microorganisms cause your chemical processing plant to
execute different processes designed to fight the microorganism attack and
return your body to homeostasis—then your body is back to normal.
As you can see, chemistry is a crucial component of microbiology. It is for this
reason that we begin the study of microorganisms with a close look at chemistry.
Everything Matters
Anything that takes up space and has mass is matter. The chair you’re sitting on
is matter. You are matter. And so are the microorganisms crawling over you and
the chair. All nonliving and living things are matter because they take up space
and have mass.
Matter is anything that takes up space and has mass. It is easy to envision
something taking up space, but what is mass?
Mass is the amount of matter a substance or an object contains. A common mis-
conception is that mass is the weight of a substance. It is true that the more there
is of a substance, the more it weighs. However, weight is the force of gravity act-
ing on mass and is calculated as weight = mass × gravity. A trip to the moon will
clarify the difference between mass and weight: You have the same mass on earth
as you do on the moon, but you weigh more on the earth than you do on the moon
because the earth has six times the gravitational force of the moon.
Chemical Elements and the Atom
Everything including you is composed of chemical elements. A chemical ele-
ment, sometimes simply referred to as an element, is a substance that cannot be
broken down into simpler substances by a chemical process. All matter is a com-
bination of chemical elements.
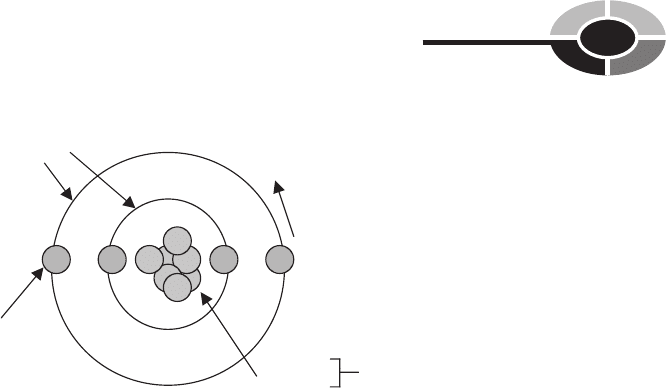
A chemical element is made up of atoms. An atom is the smallest particle of
an element; it cannot be further decomposed into smaller chemical substance
(Fig. 2-1). In the early 1800s, John Dalton developed the Atomic Theory, which
explains the relationship between an element and an atom. The Atomic Theory
CHAPTER 2 Chemical Elements of Microorganisms
24
c02_betsy.qxd 5/11/05 2:24 PM Page 24
states that an element cannot be decomposed into two or more chemical sub-
stances because the element consists of one kind of atom. The atom is also the
smallest amount of matter that can enter into a chemical reaction. You’ll learn
about chemical reactions later in this chapter.
At the center of every atom is a nucleus. The nucleus does not change spon-
taneously unless it is unstable—making it radioactive—and does not participate
in a chemical reaction. It is for this reason that the nucleus for most atoms is con-
sidered stable.
Moving around the nucleus are electrons. An electron is a negatively charged
particle that follows a path called an orbital. Electrons are the parts of an atom
that enter into a chemical reaction.
The nucleus is made up of protons and neutrons. A proton is a positively
charged particle. A neutron is a particle that does not have a charge; it is called
neutral or uncharged. The number of protons in the nucleus equals the number
of electrons in an electrically stable atom. This makes the atom neutral because
the number of positively charged particles (protons) offsets the number of neg-
atively charged particles (electrons).
An element is identified by its atomic number. The atomic number is the num-
ber of protons in the nucleus of the atom. The atomic mass (also called the atomic
weight) is slightly less than the sum of the masses of an atom’s neutrons and pro-
tons. The standard for measuring atomic mass is called a dalton, named for John
Dalton. A dalton is also known as an atomic mass unit (amu). For example, a neu-
tron has an atomic mass of 1.088 daltons. A proton has an atomic mass of 1.077
daltons. An electron has an atomic mass of 0.0005 dalton.
Atoms that have the same atomic number are classified as the same chemical
element because these atoms behave the same way. Therefore, a chemical ele-
ment consists of one or more atoms that have the same atomic number.
CHAPTER 2 Chemical Elements of Microorganisms
25
Electron
Electron shells
Proton
Neutron
Orbital
Nucleus
Fig. 2-1. An atom is the smallest particle of an element.
c02_betsy.qxd 5/11/05 2:24 PM Page 25
Atoms of elements that have the same atomic number, but different mass
numbers are called isotopes. This difference is do to a difference in number of
neutrons.
Each chemical element is identified by a one or two-letter symbol that corre-
sponds to the first letter or the first two letters in its name. For example, the sym-
bol C is used for carbon. Some chemical elements have English names while
others have Latin names. It is for this reason that symbols for some chemical
elements seem strange at first glance. Take sodium, for example. You would
think its symbol should be S, but that’s the symbol for sulfur. The symbol for
sodium is Na—the first two letters of its Latin name, natrium.
There are 92 natural chemical elements and others that scientists synthesized
(created). All of these are organized into a table called the Periodic Table (see
“A Dinner Table of Elements: The Periodic Table”). The six most abundant
chemical elements in living things are carbon, oxygen, hydrogen, nitrogen,
phosphorus and calcium. The rest are also important (see Table 2-1) and are
found in trace amounts.
CHAPTER 2 Chemical Elements of Microorganisms
26
Atomic Approximate
Element Symbol Number Atomic Weight
Calcium Ca 20 40
Carbon C 6 12
Chlorine Cl 17 35
Hydrogen H 1 1
Iodine I 53 127
Iron Fe 26 56
Magnesium Mg 12 24
Nitrogen N 7 14
Oxygen O 8 16
Phosphorus P 15 31
Potassium K 19 39
Sodium Na 11 23
Sulfur S 16 32
Table 2-1. Chemical Elements Commonly Found in All Living Things
c02_betsy.qxd 5/11/05 2:24 PM Page 26
