Bar-Meir G. Fundamentals of Compressible Fluid
Подождите немного. Документ загружается.

5.3. ISENTROPIC TABLES 81
3% (for k = 1.4). As can be observed from Figure (5.7(b)). The Mach number for
the isentropic is larger for the supersonic branch but the velocity is lower.
0
0.5
1
1.5
2
Distance (normalized distance two scales)
0
0.2
0.4
0.6
0.8
1
P / P
0 isentropic
T / T
0 isentropic
P / P
0 isothermal
T/T
0 isothermal
Comparison between the two models
k = 1 4
Fri Apr 8 15:11:44 2005
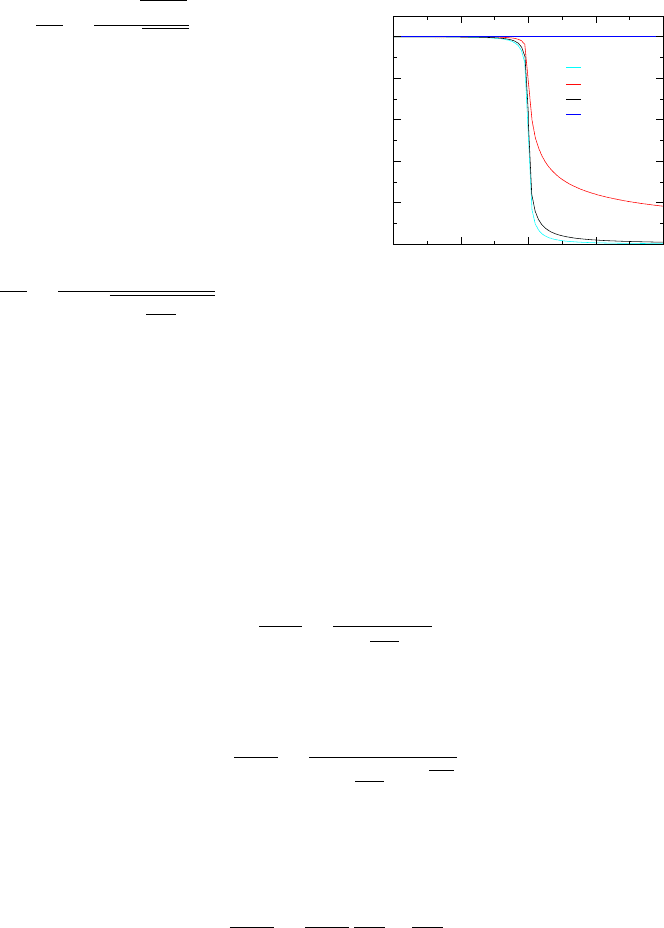
Fig. -5.8. Comparison of the pressure and tempera-
ture drop as a function of the normalized length (two
scales)
The ratio of the velocities can be ex-
pressed as
U
s
U
T
=
M
s
√
kRT
s
M
T
√
kRT
s
(5.99)
It can be noticed that temperature
in the isothermal model is constant
while temperature in the adiabatic
model can be expressed as a function
of the stagnation temperature. The
initial stagnation temperatures are al-
most the same and can be canceled
out to obtain
U
s
U
T
∼
M
s
M
T
q
1 +
k−1
2
M
s
2
(5.100)
By utilizing equation (5.100) the ve-
locity ratio was obtained and is plot-
ted in Figure (5.7(b)).
Thus, using the isentropic model results in under prediction of the actual results
for the velocity in the supersonic branch. While, the isentropic for the subsonic branch
will be over prediction. The prediction of the Mach number are similarly shown in Figure
(5.7(b)).
Two other ratios need to be examined: temperature and pressure. The initial
stagnation temperature is denoted as T
0
int
. The temperature ratio of T /T
0
int
can be
obtained via the isentropic model as
T
T
0
int
=
1
1 +
k−1
2
M
2
(5.101)
While the temperature ratio of the isothermal model is constant and equal to one (1).
The pressure ratio for the isentropic model is
P
P
0
int
=
1
¡
1 +
k−1
2
M
2
¢
k−1
k
(5.102)
and for the isothermal process the stagnation pressure varies and has to be taken into
account as the following:
P
z
P
0
int
=
P
0
∗
P
0
int
P
0
z
P
0
∗
isentropic
z}|{
P
z
P
0
z
(5.103)
82 CHAPTER 5. ISENTROPIC FLOW
where z is an arbitrary point on the nozzle. Using equations (5.88) and the isentropic
relationship, the sought ratio is provided.
Figure (5.8) shows that the range between the predicted temperatures of the two
models is very large, while the range between the predicted pressure by the two models
is relatively small. The meaning of this analysis is that transferred heat affects the
temperature to a larger degree but the effect on the pressure is much less significant.
To demonstrate the relativity of the approach advocated in this book consider the
following example.
Example 5.7:
Consider a diverging–converging nozzle made out of wood (low conductive material)
with exit area equal entrance area. The throat area ratio to entrance area is 1:4 re-
spectively. The stagnation pressure is 5[Bar] and the stagnation temperature is 27
◦
C.
Assume that the back pressure is low enough to have supersonic flow without shock
and k = 1.4. Calculate the velocity at the exit using the adiabatic model. If the nozzle
was made from copper (a go od heat conductor) a larger heat transfer occurs, should
the velocity increase or decrease? What is the maximum possible increase?
Solution
The first part of the question deals with the adiabatic model i.e. the conservation of the
stagnation properties. Thus, with known area ratio and known stagnation Potto–GDC
provides the following table:
M
T
T
0
ρ
ρ
0
A
A
?
P
P
0
A×P
A
∗
×P
0
0.14655 0.99572 0.98934 4.0000 0.98511 3.9405
2.9402 0.36644 0.08129 4.0000 0.02979 0.11915
With the known Mach number and temperature at the exit, the velocity can be cal-
culated. The exit temperature is 0.36644 × 300 = 109.9K. The exit velocity, then,
is
U = M
√
kRT = 2.9402
√
1.4 ×287 × 109.9 ∼ 617.93[m/sec]
Even for the isothermal model, the initial stagnation temperature is given as
300K. Using the area ratio in Figure (5.6) or using the Potto–GDC obtains the following
table
M
T
T
0
ρ
ρ
0
A
A
?
P
P
0
A×P
A
∗
×P
0
1.9910 1.4940 0.51183 4.0000 0.12556 0.50225
The exit Mach number is known and the initial temperature to the throat temperature
ratio can be calculated as the following:
T
0
ini
T
0
∗
=
1
1 +
k−1
2
1
k
=
1
1 +
k−1
k
= 0.777777778
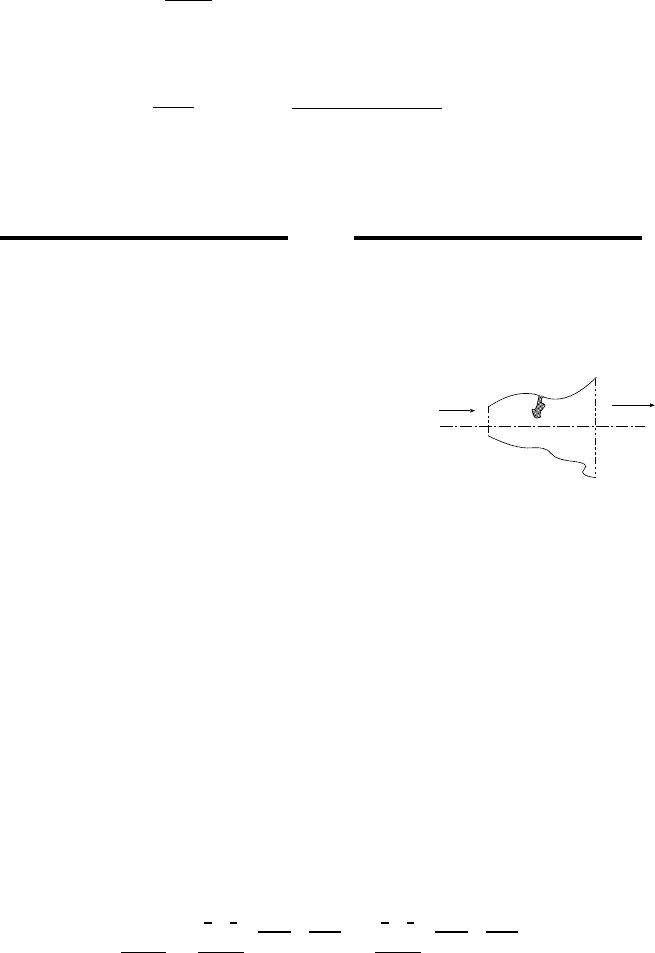
5.4. THE IMPULSE FUNCTION 83
Thus the stagnation temperature at the exit is
T
0
ini
T
0
exit
= 1.4940/0.777777778 = 1.921
The exit stagnation temperature is 1.92 × 300 = 576.2K. The exit velocity can be
determined by utilizing the following equation
U
exit
= M
√
kRT = 1.9910
√
1.4 ×287 × 300.0 = 691.253[m/sec]
As was discussed before, the velocity in the copper nozzle will be larger than
the velocity in the wood nozzle. However, the maximum velocity cannot exceed the
691.253[m/sec]
End solution
5.4 The Impulse Function
5.4.1 Impulse in Isentropic Adiabatic Nozzle
x-direction
Fig. -5.9. Schematic to explain the
significances of the Impulse function.
One of the functions that is used in calculating the
forces is the Impulse function. The Impulse func-
tion is denoted here as F , but in the literature some
denote this function as I. To explain the motiva-
tion for using this definition consider the calculation
of the net forces that acting on section shown in
Figure (5.9). To calculate the net forces acting in
the x–direction the momentum equation has to be
applied
F
net
= ˙m(U
2
− U
1
) + P
2
A
2
− P
1
A
1
(5.104)
The net force is denoted here as F
net
. The mass conservation also can be applied to
our control volume
˙m = ρ
1
A
1
U
1
= ρ
2
A
2
U
2
(5.105)
Combining equation (5.104) with equation (5.105) and by utilizing the identity in
equation (5.42) results in
F
net
= kP
2
A
2
M
2
2
− kP
1
A
1
M
1
2
+ P
2
A
2
− P
1
A
1
(5.106)
Rearranging equation (5.106) and dividing it by P
0
A
∗
results in
F
net
P
0
A
∗
=
f(M
2
)
z}|{
P
2
A
2
P
0
A
∗
f(M
2
)
z }| {
¡
1 + kM
2
2
¢
−
f(M
1
)
z}|{
P
1
A
1
P
0
A
∗
f(M
1
)
z }| {
¡
1 + kM
1
2
¢
(5.107)
84 CHAPTER 5. ISENTROPIC FLOW
Examining equation (5.107) shows that the right hand side is only a function
of Mach number and specific heat ratio, k. Hence, if the right hand side is only a
function of the Mach number and k than the left hand side must be function of only
the same parameters, M and k. Defining a function that depends only on the Mach
number creates the convenience for calculating the net forces acting on any device.
Thus, defining the Impulse function as
F = P A
¡
1 + kM
2
2
¢
(5.108)
In the Impulse function when F (M = 1) is denoted as F
∗
F
∗
= P
∗
A
∗
(1 + k) (5.109)
The ratio of the Impulse function is defined as
F
F
∗
=
P
1
A
1
P
∗
A
∗
¡
1 + kM
1
2
¢
(1 + k)
=
1
P
∗
P
0
|{z}
(
2
k+1
)
k
k−1
see function (5.107)
z }| {
P
1
A
1
P
0
A
∗
¡
1 + kM
1
2
¢
1
(1 + k)
(5.110)
This ratio is different only in a coefficient from the ratio defined in equation (5.107)
which makes the ratio a function of k and the Mach number. Hence, the net force is
F
net
= P
0
A
∗
(1 + k)
µ
k + 1
2
¶
k
k−1
µ
F
2
F
∗
−
F
1
F
∗
¶
(5.111)
To demonstrate the usefulness of the this function consider a simple situation of
the flow through a converging nozzle
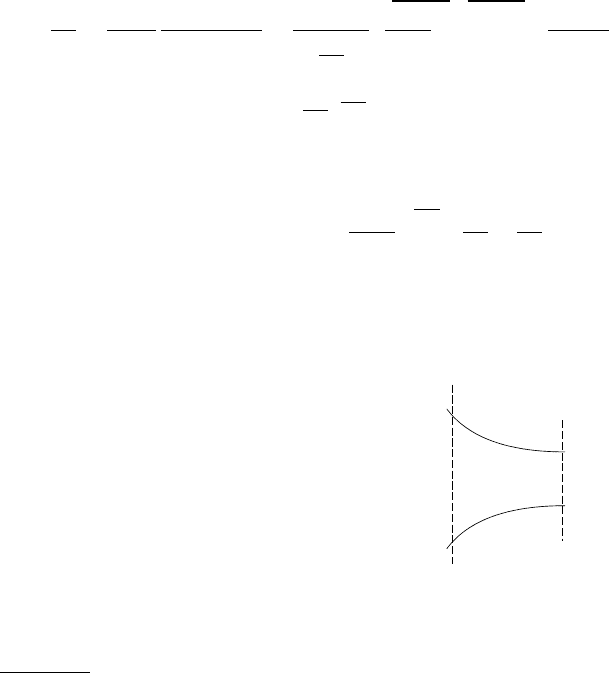
Example 5.8:
1
2
˙m = 1[kg/sec]
A
1
= 0.009m
2
T
0
= 400K
A
2
= 0.003m
2
P
2
= 50[Bar]
Fig. -5.10. Schematic of a flow of a compressible sub-
stance (gas) through a converging nozzle for example
(5.8)
Consider a flow of gas into a con-
verging nozzle with a mass flow
rate of 1[kg/sec] and the en-
trance area is 0.009[m
2
] and the
exit area is 0.003[m
2
]. The stag-
nation temperature is 400K and
the pressure at point 2 was mea-
sured as 5[Bar ] Calculate the net
force acting on the nozzle and
pressure at point 1.
Solution
The solution is obtained by getting the data for the Mach number. To obtained the
5.4. THE IMPULSE FUNCTION 85
Mach number, the ratio of P
1
A
1
/A
∗
P
0
is needed to be calculated. To obtain this ratio
the denominator is needed to be obtained. Utilizing Fliegner’s equation (5.51), provides
the following
A
∗
P
0
=
˙m
√
RT
0.058
=
1.0 ×
√
400 ×287
0.058
∼ 70061.76[N]
and
A
2
P
2
A
∗
P
0
=
500000 ×0.003
70061.76
∼ 2.1
M
T
T
0
ρ
ρ
0
A
A
?
P
P
0
A×P
A
∗
×P
0
F
F
∗
0.27353 0.98526 0.96355 2.2121 0.94934 2.1000 0.96666
With the area ratio of
A
A
?
= 2.2121 the area ratio of at point 1 can be calculated.
A
1
A
?
=
A
2
A
?
A
1
A
2
= 2.2121 ×
0.009
0.003
= 5.2227
And utilizing again Potto-GDC provides
M
T
T
0
ρ
ρ
0
A
A
?
P
P
0
A×P
A
∗
×P
0
F
F
∗
0.11164 0.99751 0.99380 5.2227 0.99132 5.1774 2.1949
The pressure at point 1 is
P
1
= P
2
P
0
P
2
P
1
P
0
= 5.0times0.94934/0.99380 ∼ 4.776[Bar]
The net force is obtained by utilizing equation (5.111)
F
net
= P
2
A
2
P
0
A
∗
P
2
A
2
(1 + k)
µ
k + 1
2
¶
k
k−1
µ
F
2
F
∗
−
F
1
F
∗
¶
= 500000 ×
1
2.1
× 2.4 × 1.2
3.5
× (2.1949 − 0.96666) ∼ 614[kN]
End solution
5.4.2 The Impulse Function in Isothermal Nozzle
Previously Impulse function was developed in the isentropic adiabatic flow. The same
is done here for the isothermal nozzle flow model. As previously, the definition of the
86 CHAPTER 5. ISENTROPIC FLOW
Impulse function is reused. The ratio of the impulse function for two points on the
nozzle is
F
2
F
1
=
P
2
A
2
+ ρ
2
U
2
2
A
2
P
1
A
1
+ ρ
1
U
1
2
A
1
(5.112)
Utilizing the ideal gas model for density and some rearrangement results in
F
2
F
1
=
P
2
A
2
P
1
A
1
1 +
U
2
2
RT
1 +
U
1
2
RT
(5.113)
Since U
2
/RT = kM
2
and the ratio of equation (5.86) transformed equation into
(5.113)
F
2
F
1
=
M
1
M
2
1 + kM
2
2
1 + kM
1
2
(5.114)
At the star condition (M = 1) (not the minimum point) results in
F
2
F
∗
=
1
M
2
1 + kM
2
2
1 + k
(5.115)
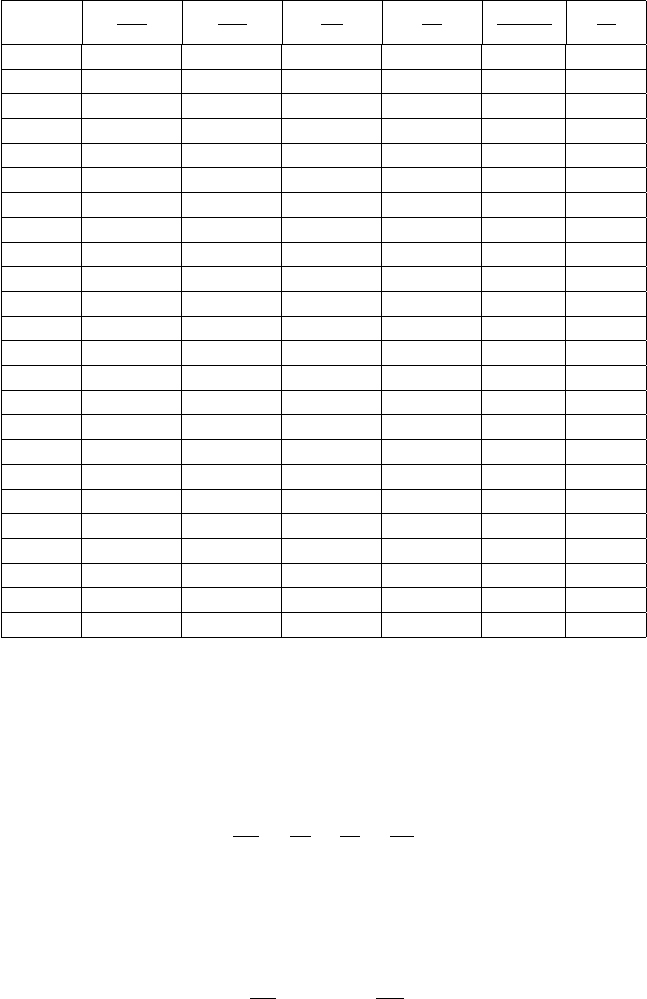
5.5 Isothermal Table
Table -5.3. Isothermal Table
M
T
0
T
0
?
P
0
P
0
?
A
A
?
P
P
?
A×P
A
∗
×P
0
F
F
∗
0.00 0.52828 1.064 5.0E + 5 2.014 1.0E+6 4.2E+5
0.05 0.52921 1.064 9.949 2.010 20.00 8.362
0.1 0.53199 1.064 5.001 2.000 10.00 4.225
0.2 0.54322 1.064 2.553 1.958 5.000 2.200
0.3 0.56232 1.063 1.763 1.891 3.333 1.564
0.4 0.58985 1.062 1.389 1.800 2.500 1.275
0.5 0.62665 1.059 1.183 1.690 2.000 1.125
0.6 0.67383 1.055 1.065 1.565 1.667 1.044
0.7 0.73278 1.047 0.99967 1.429 1.429 1.004
0.8 0.80528 1.036 0.97156 1.287 1.250 0.98750
0.9 0.89348 1.021 0.97274 1.142 1.111 0.98796
1.00 1.000 1.000 1.000 1.000 1.000 1.000
1.10 1.128 0.97376 1.053 0.86329 0.90909 1.020
1.20 1.281 0.94147 1.134 0.73492 0.83333 1.047
5.6. THE EFFECTS OF REAL GASES 87
Table -5.3. Isothermal Table (continue)
M
T
0
T
0
?
P
0
P
0
?
A
A
?
P
P
?
A×P
A
∗
×P
0
F
F
∗
1.30 1.464 0.90302 1.247 0.61693 0.76923 1.079
1.40 1.681 0.85853 1.399 0.51069 0.71429 1.114
1.50 1.939 0.80844 1.599 0.41686 0.66667 1.153
1.60 2.245 0.75344 1.863 0.33554 0.62500 1.194
1.70 2.608 0.69449 2.209 0.26634 0.58824 1.237
1.80 3.035 0.63276 2.665 0.20846 0.55556 1.281
1.90 3.540 0.56954 3.271 0.16090 0.52632 1.328
2.00 4.134 0.50618 4.083 0.12246 0.50000 1.375
2.50 9.026 0.22881 15.78 0.025349 0.40000 1.625
3.000 19.41 0.071758 90.14 0.00370 0.33333 1.889
3.500 40.29 0.015317 7.5E + 2 0.000380 0.28571 2.161
4.000 80.21 0.00221 9.1E + 3 2.75E−5 0.25000 2.438
4.500 1.5E + 2 0.000215 1.6E + 5 1.41E−6 0.22222 2.718
5.000 2.8E + 2 1.41E−5 4.0E + 6 0.0 0.20000 3.000
5.500 4.9E + 2 0.0 1.4E + 8 0.0 0.18182 3.284
6.000 8.3E + 2 0.0 7.3E + 9 0.0 0.16667 3.569
6.500 1.4E + 3 0.0 5.3E+11 0.0 0.15385 3.856
7.000 2.2E + 3 0.0 5.6E+13 0.0 0.14286 4.143
7.500 3.4E + 3 0.0 8.3E+15 0.0 0.13333 4.431
8.000 5.2E + 3 0.0 1.8E+18 0.0 0.12500 4.719
8.500 7.7E + 3 0.0 5.4E+20 0.0 0.11765 5.007
9.000 1.1E + 4 0.0 2.3E+23 0.0 0.11111 5.296
9.500 1.6E + 4 0.0 1.4E+26 0.0 0.10526 5.586
10.00 2.2E + 4 0.0 1.2E+29 0.0 0.100000 5.875
5.6 The effects of Real Gases
To obtained expressions for non–ideal gas it is commonly done by reusing the ideal gas
model and introducing a new variable which is a function of the gas properties like the
critical pressure and critical temperature. Thus, a real gas equation can be expressed in
equation (4.22). Differentiating equation (4.22) and dividing by equation (4.22) yields
dP
P
=
dz
z
+
dρ
ρ
+
dT
T
(5.116)
Again, Gibb’s equation (5.27) is reused to related the entropy change to the change
in thermodynamics properties and applied on non-ideal gas. Since ds = 0 and utilizing
the equation of the state dh = dP/ρ. The enthalpy is a function of the temperature
and pressure thus, h = h(T, P ) and full differential is
dh =
µ
∂h
∂T
¶
P
dT +
µ
∂h
∂P
¶
T
dP (5.117)

88 CHAPTER 5. ISENTROPIC FLOW
The definition of pressure specific heat is C
p
≡
∂h
∂T
and second derivative is Maxwell
relation hence,
µ
∂h
∂P
¶
T
= v − T
µ
∂s
∂T
¶
P
(5.118)
First, the differential of enthalpy is calculated for real gas equation of state as
dh = C
p
dT −
µ
T
Z
¶µ
∂z
∂T
¶
P
dP
ρ
(5.119)
Equations (5.27) and (4.22) are combined to form
ds
R
=
C
p
R
dT
T
− z
·
1 +
µ
T
Z
¶µ
∂z
∂T
¶
P
¸
dP
P
(5.120)
The mechanical energy equation can be expressed as
Z
d
µ
U
2
2
¶
= −
Z
dP
ρ
(5.121)
At the stagnation the definition requires that the velocity is zero. To carry the inte-
gration of the right hand side the relationship between the pressure and the density has
to be defined. The following p ower relationship is assumed
ρ
ρ
0
=
µ
P
P
0
¶
1
n
(5.122)
Notice, that for perfect gas the n is substituted by k. With integration of equation
(5.121) when using relationship which is defined in equation (5.122) results
U
2
2
=
Z
P
1
P
0
dP
ρ
=
Z
P
P
0
1
ρ
0
µ
P
0
P
¶
1
n
dP (5.123)
Substituting relation for stagnation density (4.22) results
U
2
2
=
Z
P
P
0
z
0
RT
0
P
0
µ
P
0
P
¶
1
n
dP (5.124)
For n > 1 the integration results in
U =
v
u
u
t
z
0
RT
0
2n
n −1
"
1 −
µ
P
P
0
¶
(
n−1
n
)
#
(5.125)
For n = 1 the integration becomes
U =
s
2z
0
RT
0
ln
µ
P
0
P
¶
(5.126)
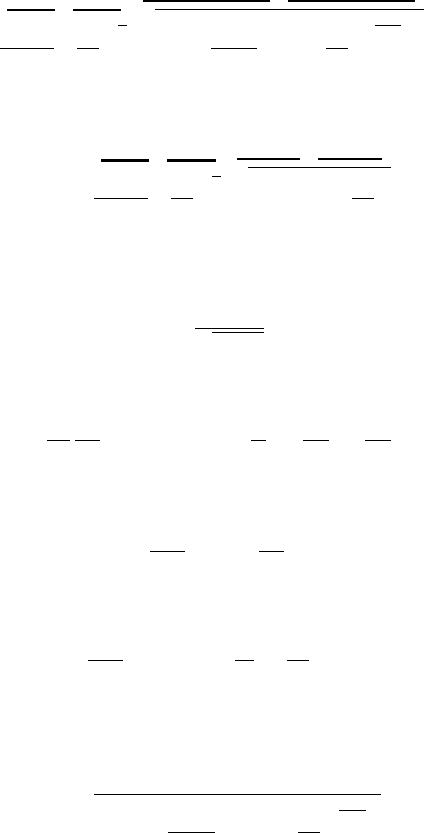
5.6. THE EFFECTS OF REAL GASES 89
It must be noted that n is a function of the critical temperature and critical pressure.
The mass flow rate is regardless to equation of state as following
˙m = ρ
∗
A
∗
U
∗
(5.127)
Where ρ
∗
is the density at the throat (assuming the chocking condition) and A
∗
is the
cross area of the throat. Thus, the mass flow rate in our properties
˙m = A
∗
ρ
∗
z }| {
P
0
z
0
RT
0
µ
P
P
0
¶
1
n
U
∗
z }| {
v
u
u
t
z
0
RT
0
2n
n −1
"
1 −
µ
P
P
0
¶
(
n−1
n
)
#
(5.128)
For the case of n = 1
˙m = A
∗
ρ
∗
z }| {
P
0
z
0
RT
0
µ
P
P
0
¶
1
n
U∗∗
z }| {
s
2z
0
RT
0
ln
µ
P
0
P
¶
(5.129)
The Mach number can be obtained by utilizing equation (4.37) to defined the Mach
number as
M =
U
√
znRT
(5.130)
Integrating equation (5.120) when ds = 0 results
Z
T
2
T
1
C
p
R
dT
T
=
Z
P
2
P
1
z
µ
1 +
µ
T
Z
¶µ
∂z
∂T
¶
P
dP
P
¶
(5.131)
To carryout the integration of equation (5.131) looks at Bernnolli’s equation which is
Z
dU
2
2
= −
Z
dP
ρ
(5.132)
After integration of the velocity
dU
2
2
= −
Z
P/P
0
1
ρ
0
ρ
d
µ
P
P
0
¶
(5.133)
It was shown in Chapter (4) that (4.36) is applicable for some ranges of relative
temperature and pressure (relative to critical temperature and pressure and not the
stagnation conditions).
U =
v
u
u
t
z
0
RT
0
µ
2n
n −1
¶
"
1 −
µ
P
P
0
¶
n−1
n
#
(5.134)
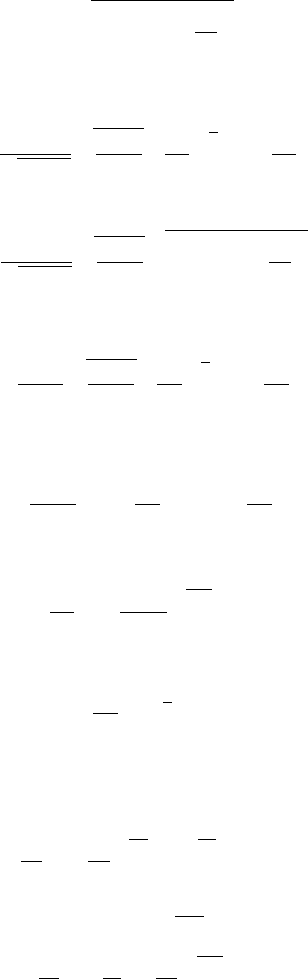
90 CHAPTER 5. ISENTROPIC FLOW
When n = 1 or when n → 1
U =
s
2z
0
RT
0
ln
µ
P
0
P
¶
(5.135)
The mass flow rate for the real gas ˙m = ρ
∗
U
∗
A
∗
˙m =
A
∗
P
0
√
z
0
RT
0
r
2n
n −1
µ
P
∗
P
0
¶
1
n
·
1 −
P
∗
P
0
¸
(5.136)
And for n = 1
˙m =
A
∗
P
0
√
z
0
RT
0
r
2n
n −1
s
2z
0
RT
0
ln
µ
P
0
P
¶
(5.137)
Fliegner’s number in this case is
F n =
˙mc
0
A
∗
P
0
r
2n
n −1
µ
P
∗
P
0
¶
1
n
·
1 −
P
∗
P
0
¸
(5.138)
Fliegner’s number for n = 1 is
F n =
˙mc
0
A
∗
P
0
= 2
µ
P
∗
P
0
¶
2
− ln
µ
P
∗
P
0
¶
(5.139)
The critical ratio of the pressure is
P
∗
P
0
=
µ
2
n + 1
¶
n
n−1
(5.140)
When n = 1 or more generally when n → 1 this is a ratio approach
P
∗
P
0
=
√
e (5.141)
To obtain the relationship between the temperature and pressure, equation (5.131)
can be integrated
T
0
T
=
µ
P
0
P
¶
R
C
p
[
z+T
(
∂z
∂T
)
P
]
(5.142)
The power of the pressure ratio is approaching
k−1
k
when z approaches 1. Note that
T
0
T
=
³
z
0
z
´
µ
P
0
P
¶
1−n
n
(5.143)
