Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

OH
N
OH
Bu
t
-
Ph
S
Me
Ph
S
Me
O
NO
2
t
-Bu
NOH
t
-Bu
HO
Ph
N
OH
1 mol% VO(acac)
2
L*, H
2
O
2,
CH
2
Cl
2
*
11
1312
L* = 11 94% (70% ee)
24
L* = 12 92% (78% ee)
25
L* = 13 83% (83% ee)
26
ð8:6Þ
Vetter and Berkessel later improved this reaction by changing the ligand to 12,
which afforded 78% ee (Eq. (8.6)) [30]. Further improvement of this protocol was
reported by Katsuki and coworkers [31], who used ligand 13 to obtain 83% ee in
the oxidation of thioanisole to sulfoxide (Eq. (8.6)). A further increase in the
enantioselectivity with ligand 13 was obtained with methanol as an additive (2%
methanol in methylene chloride) [31]. With this protocol ees up to 93% were
obtained for aryl methyl sulfides (Eq. (8.7)). In all of the reactions, except for
Ar ¼ p-NO
2
C
6
H
4
, only traces of sulfone was formed. The latter substrate gave 10%
sulfone.
Ar
S
Me
Ar
S
Me
O
1 mol% VO(acac)
2
3, H
2
O
2,
CH
2
Cl
2
with 2% of MeOH
*
Ar = Ph 81% (88% ee)
Ar =
p
-ClC
6
H
4
83% (88% ee)
Ar = 2-naphtyl 94% (93% ee)
ð8:7Þ
More recently, Bolm and coworkers extended their original work on the Schiff-
base ligands to iron-catalyzed reactions [5, 32]. With the use of ligand (S)-14 they
obtained high ee in the oxidation of various sulfides. In the original report the yields
of sulfoxide were moderate (up to 44%), and sulfoxides could be obtained in up to
90% ee. The reaction was later improved by the addition of additives, and with
benzoic acid as additive methyl tolyl sulfide was oxidized to the (S)-sulfoxide in 78%
yield and 92% ee [32b]. The highest ee obtained with the improved procedure was
96%, but then the yield was more moderate.
8.2 Oxidation of Sulfides to Sulfoxides
j
283
p
-Me-C
6
H
4
S
4 mol% of (S)-14
2 mol% of Fe(acac)
3
1 mol% of p-MeOC
6
H
4
CO
2
H
H
2
O
2
(1.2 equiv.)
CHCl
3
, 0
o
C
p-Me-C
6
H
4
S
O
*
78% (92% ee)
I
I
OHN
OH
(
S
)
-14
(S)
ð8:8Þ
Jackson and coworkers [33] developed immobilized Schiff-base ligands inspired
by those used by Bolm. A peptide Schiff-base library with ligands 15 bound to solid
support was investigated, where two amino acids (AA1 and AA2) and the salicy-
laldehyde were varied. A library of 72 ligands was prepared using six different
salicylaldehydes, six different amino acids as aminoacid 1 (AA1) and two different
aminoacids as aminoacid 2 (AA2). Screening of these ligands in the VO(acac)
2
-
catalyzed H
2
O
2
oxidation of sulfides in CH
2
Cl
2
gave only a moderate enantioselec-
tivity of 11% for thioanisole with the best ligand (R
1
¼ Ph, R
2
¼ H). Screening the
ligands with Ti(O
i
Pr)
4
as catalyst gave a better result, and thioanisole afforded
the sulfoxide in quantitative yield in 64% ee with ligand 16. The best result with
this ligand was obtained with 2-naphthyl methyl sulfide, which gave 72% ee in a high
yield (Eq. (8.9)).
S
Me
S
Me
5 mol% Ti(O-i-Pr)
4
ligand 16
30% H
2
O
2
, CH
2
Cl
2
87%
(
72% ee
)
O
ð8:9Þ
O
O
N
H
R
1
O
N
OH
R
2
OH
O
O
N
H
Ph
O
N
OH
Me
OH
X
15
16
Bu
t
-Bu
t
-
A vanadium-catalyzed enantioselective sulfoxidation using related Schiff-base
ligands was later developed by the Jackson group. In this reaction, selective oxidation
284
j
8 Selective Oxidation of Amines and Sulfides
of the minor enantiomer of the sulfoxide to sulfone occurs, which improves the ee
of the product. For example, methyl tolyl sulfide was oxidized to its (R)-sulfoxide in
99.5% ee with a yield of 82% using ligand (R)-17 [34].
(
R
)-17ofmol%1.5
VO(acac)ofmol%1.0
2
H
2
O
2
equv)(1.2
CHCl
3,
0
o
C
(
R
)
ee)(99.5%82%
I
I
OHN
OH
(
R
)-17
p
-Me-C
6
H
4
S
O
p
-Me-C
6
H
4
S
*
ð8:10Þ
Chiral Mn(III)(salen) complexes have been used as catalysts in the oxidation of
sulfides to sulfoxides by 30% aqueous H
2
O
2
in acetonitrile [35]. The use of 2–3 mol%
of catalyst led to an efficient reaction with enantioselectivities up to 68% ee. The use
of Ti(salen) complexes as catalysts and aqueous H
2
O
2
in methanol afforded sulf-
oxides in 76% ee from the oxidation of sulfides [36]. Katsuki has recently improved
these types of catalysts with other metals for H
2
O
2
-based sulfoxidation and obtained
highly efficient reactions with excellent enantioselectivity [37, 38]. With a chiral
Al(salalene) complex as catalyst, methyl phenyl sulfide was oxidized to the corre-
sponding sulfoxide in 90% yield and 98% ee [37a]. The sulfoxidation was accompa-
nied by formation of 9% of sulfone. This overoxidation was shown to account for the
high ee by removing some of the minor enantiomer of the sulfoxide. The reaction was
subsequently improved to give 99% ee under solvent-free or highly concentrated
conditions [37b]. An iron-based catalyst, Fe(salan) was used for the enantioselective
oxidation of various sulfides in high yields and enantioselectivities up to 96% ee.
Platinum-catalyzed asymmetric sulfoxidation of thioethers with hydrogen peroxide
in water was reported to give up to 88% ee.(R)-BINAP was used as chiral ligand on the
metal [39].
Oxidation of sulfides in the presence of electron-rich double bonds is problematic
with many of the traditional oxidants such as MCPBA, NaIO
4
, and oxone because of
interference with double bond oxidation (e.g., epoxidation). Koo and coworkers [40]
addressed this problem and studied the selective oxidation of allylic sulfides having
multiple alkyl substituents. They tested various stoichiometric oxidants and a
number of catalytic reactions with 30% aqueous H
2
O
2
as the oxidant. Of all the
oxidation systems tested for the sulfoxidation, they found that the use of LiNbMoO
6
as catalyst with H
2
O
2
as the oxidant gave the best result. With this system no
epoxidation took place and a reasonably good selectivity for sulfoxide over sulfone
was obtained (Table 8.2).
Lanthanides as Catalysts Catalytic amounts of scandium triflate (Sc(OTf)
3
) was
found to greatly increase the rate of oxidation of sulfides by 60% H
2
O
2
(Table 8.3) [41].
8.2 Oxidation of Sulfides to Sulfoxides
j
285
The reaction is run at room temperature in methylene chloride containing 10% of
ethanol. The reaction shows quite a high selectivity for sulfoxide, with only 2–4%
sulfones being formed.
Table 8.2 Selective oxidation of allylic sulfides with electron-rich double bonds.
R
S
R'
R
S
R'
O
30% aq. H
2
O
2
0
o
C
LiNbMoO
6
Entry Sulfide % Yield
Sulfoxide Sulfone
1
SPh
77 14
2
SPh
80 4
3
SPh
HO
75 12
4
SPh
TBDMSO
82 12
5
S
54 8
Table 8.3 Oxidation of sulfides in the presence of catalytic amounts of scandium triflate.
R
S
R'
R
S
R'
O
60% aq. H
2
O
2
CH
2
Cl
2
/10%EtOH
room temp
cat. Sc(OTf)
3
Entry Substrate Reaction time (h) % Yield
Sulfoxide Sulfone
1
Ph
S
Me
3944
2
Ph
S
2982
3
p
-Br-C
6
H
4
S
Me
3982
4
C
6
H
4
S
Me
p
-MeO-
1.3 98 2
286
j
8 Selective Oxidation of Amines and Sulfides
N
N
NH
N
CH
2
O
O
Me
Me
OH
OH
OH
CH
2
PO
O
O
-
PO
O
O
-
R
FAD
N
N
NH
N
CH
2
O
O
Me
Me
OH
OH
OH
CH
2
PO
O
O
-
PO
O
O
-
R
FADH
2
+ 2H
+
+ 2e
-
H
H
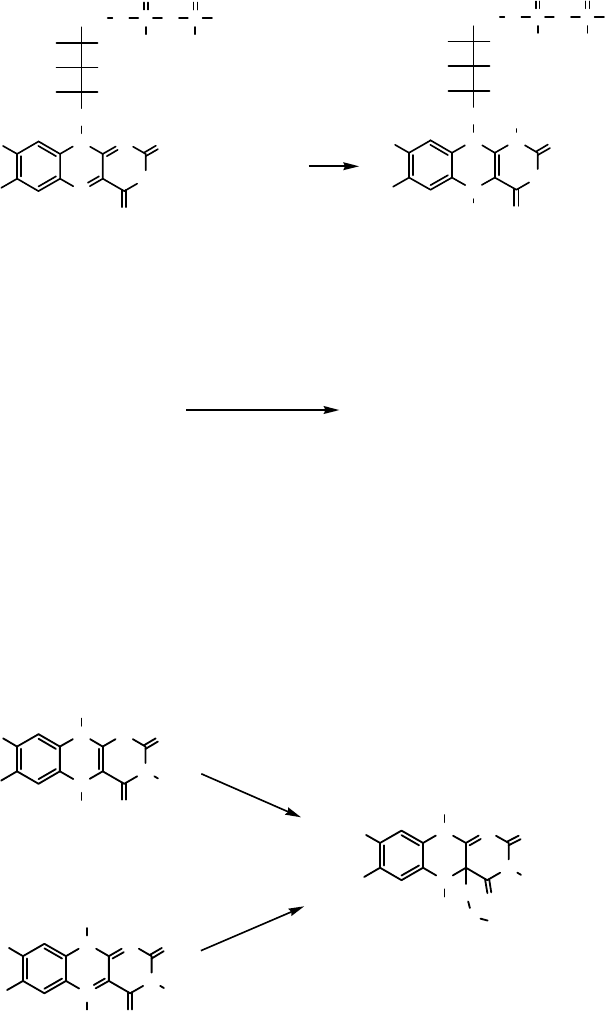
Figure 8.1 The FAD/FADH
2
redox system is a cofactor in flovoenzymes (R ¼ adenosine).
The reaction was applied to the oxidation of various cysteine derivatives to their
corresponding sulfoxides (Eq. (8.11)).
Boc-Phe-Cys(Me)Ala-OAll
20 mol% Sc(OTf)
3
60% aq. H
2
O
2
15 min
Boc-Phe-Cys(O)(Me)Ala-OAll
100%
ð8:11Þ
Flavins as Catalysts Flavins are organic molecules that are part of the FADH
2
cofactor of flavoenzymes (Figure 8.1). In FAD-containing monooxygenases
(FADMOs), molecular oxygen is activated to generate a flavin hydroperoxide.
Model compounds of the natural flavins were studied by Bruice in the late 1970s
[42]. In these studies N,N,N,-3,5,10-trialkylated flavins 16 and 17 were used. It was
demonstrated that reactive flavin hydroperoxides can be generated from the reduced
form 16 and molecular oxygen or from the oxidized form 17 and H
2
O
2
(Scheme 8.2).
N
N
N
N
Me
O
OEt
+
Me
Me Me
N
N
N
H
N
Me
O
OEt
Me
Me Me
N
N
N
N
Me
O
O
Et
Me
Me Me
O
O
H
16
17
18
O
2
H
2
O
2
Scheme 8.2
8.2 Oxidation of Sulfides to Sulfoxides
j
287
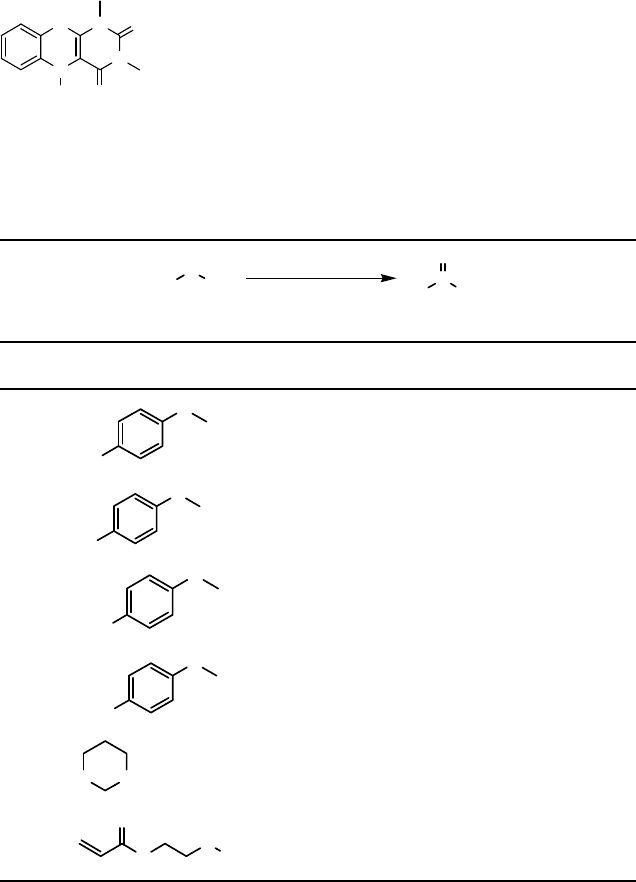
Stoichiometric oxidation reactions with hydroperoxide 18 were studied, and it was
found that 18 oxidizes sulfides to sulfoxides in a highly selective manner [42, 43].
It was later demonstrated that these flavins can participate as catalysts for the H
2
O
2
oxidation of sulfides to sulfoxides [44, 45].
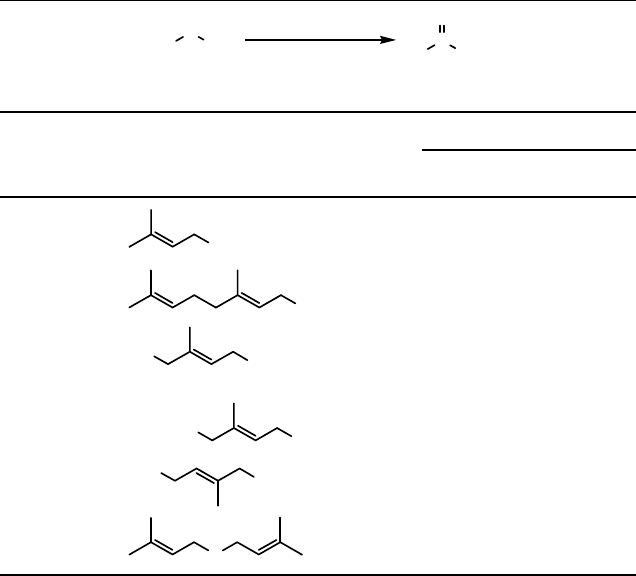
More recently, a modification of the structure of these flavins gave more efficient
and robust organocatalysts for the H
2
O
2
-based sulfoxidations [46]. These new flavin
catalysts (19) are superior to the previous natural-based flavin catalysts and have the
advantage that they also give excellent results for the oxidation of tertiary amines to
amine oxides [47] (see Section 8.3.2).
N
H
N
N
N
O
Et O
19
Various thioethers were oxidized with the use of flavin 19 as the catalyst (Table 8.4)
[46]. Only sulfoxide was formed and no overoxidation to sulfone could be detected.
Table 8.4 Oxidation of thioethers using flavin 19 as catalyst.
R
S
R'
R
S
R'
O
~1.5 mol% of 19
30% aq. H
2
O
2
MeOH, room temp
Entry Substrate Mol% Reaction time % Yield of sulfoxide
1
Me
S
1.8 1 h 100
2
Br
S
1.6 2 h 40 min 96
3
H
2
N
S
1.3 23 min 99
4
MeO
S
1.6 45 min 92
5
SS
1.7 20 min 99
6
O
S
O
Me
1.1 30 min 99
288
j
8 Selective Oxidation of Amines and Sulfides
The structure of the flavin was studied, and the new flavin structures (N,N,N-1,3,5-
trialkyl) were compared with those previously used (N,N,N-3,5,10-trialkyl). It was
found that the new flavins were between one and two orders of magnitude faster than
the previously used flavins.
The flavin-catalyzed sulfoxidation was later extended to allylic and vinylic sulfides.
It was found that the oxidation of allylic sulfides having electron-rich double bonds
proceed with an exceptional selectivity for sulfoxidation (Table 8.5) [48]. Sulfone
formation was depressed below detection (<0.5%) and only in one single case could
sulfone be observed (1.5% relative yield, for entry 6). No epoxide could be detected.
The mechanism of the flavin-catalyzed oxidation of sulfides by hydrogen peroxide
is shown in Scheme 8.3.
The r eaction is initiat ed by reaction of catalyst 19 with molecular oxygen to give
flavin hydroperoxid 20. Once in the cycle, this hydroperoxide can be regenerated by
H
2
O
2
. The hydroperoxide 20 transfers an oxygen to the sulfide via t he hydrogen-
bonded transition state 21 to give sulfoxide and hydroxyflavin intermediate 22.
Table 8.5 Flavin-catalyzed sulfoxidation of sulfides.
R
S
R'
R
S
R'
O
2 mol% of 19
30% aq. H
2
O
2
MeOH, room temp
2.5 - 3 h
Entry Sulfide % Yield
Sulfoxide Sulfone
a)
1
SPh
92 n.d.
2
SPh
76 n.d.
3
SPh
HO
77 n.d.
4
SPh
SPh
TBDMSO
87 n.d.
5
SPh
AcO
96 n.d.
6
S
85 1.3
a) n.d. ¼ not detected.
8.2 Oxidation of Sulfides to Sulfoxides
j
289
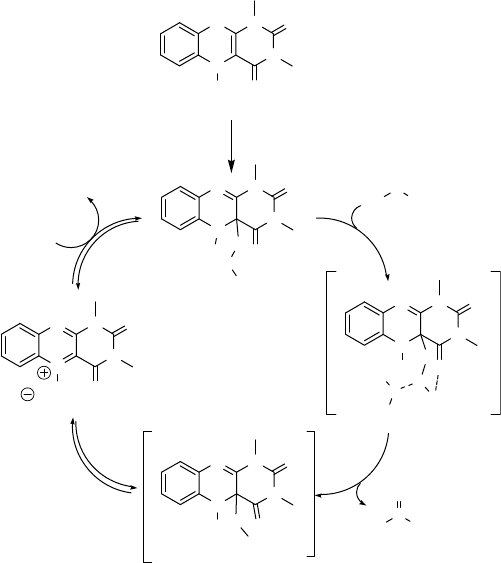
Elimination of OH
from 22 produces the aromatic 1,4-diazine 23. The latter
species, which becomes the catalytic intermediate, reac ts wit h h ydrogen peroxide to
regenerate the catalytic flavin hydroperoxide. T he advantage of th e N,N,N-1,3,5-
trialkyl flavin system over the N,N,N-3,5,10-trialkylated analogs is that the elim-
ination of OH
(22 ! 23) is fast because of aromatization. In the N,N,N-3,5,10-
trialkylated system this step is slow and has been found to be the rate-determining
step [44].
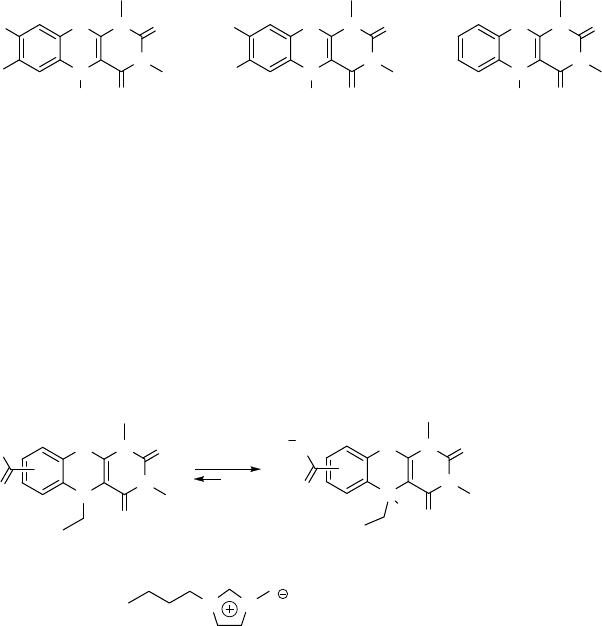
The r edox properties of eight different N,N,N -1,3,5-t rialkyl ated flavins and
their activity as redox cat alysts for the oxidation of sulfides were stu died [49]. Th e
redox potentials (E
0
) were determined by cyclic voltammetry, and for the eight
flavins studied E
0
varied from 0.414 to 0.162 V. There was a linear free-
energy relationship betw een the oxidation potentials and the Hammett s value.
The redox potentials of t he flavins also c orrelated well with their efficiency
as catalysts in the H
2
O
2
oxidation of methyl p-tolyl sulfide. I nterestingly, it was
found that the electron rich 7,8-dimethyl derivative 24 (E
0
¼0.414) was
oxidation sensitive
N
N
N
N
O
Et
O
O
O
N
H
N
N
N
O
Et O
O
2
N
N
N
N
O
Et
O
O
H
O
S
R
R'
S
R'R
O
N
N
N
N
O
Et
O
O
H
N
N
N
N
O
Et O
OH
H
2
O
2
H
2
O
H
20
19
21
22
23
S
R'
R
Scheme 8.3
290
j
8 Selective Oxidation of Amines and Sulfides
N
H
N
N
N O
Et O
N
H
N
N
N O
Et O
N
H
N
N
N O
Et O
192524
Me
Me
F
F
and underwent degradation, whereas the 7,8-difluoro derivative 25 (E
0
¼0.207) was
very stable. The latter derivative showed an induction period in the oxidation of p-tolyl
sulfide with H
2
O
2
due to the slow generation of hydroperoxide (cf. 19 ! 20 in
Scheme 8.3). Once formed, the difl uoro flavin 25 is a highly efficient and robust
catalyst. Catalyst 19 is also quite stable, and furthermore it does not show any
detectable induction period, which is consistent with the lower redox potential
(0.305) compared to that of 25 (0.207).
A derivative of the N,N,N-1,3,5-trialkylated flavins with a carboxylate group in
either the 7 or 8 position (26) was studied as a recyclable catalyst for H
2
O
2
-based
sulfoxidation in ionic liquid [BMIm]PF
6
[50]. It was found that flavin 26 is a highly
efficient catalyst for the
N
H
N
N
NO
O
O
HO
26
N
H
N
N
NO
O
O
O
H
+
NN
PF
6
[BMIm]PF
6
H
2
O
2
oxidation of various sulfides to sulfoxides. The sulfoxides were obtained in
good to high yields and high selectivity without any detectable overoxidation to
sulfone. More importantly, the flavin catalyst 26 in the ionic liquid was recycled up to
seven times in the sulfoxidation of some representative sulfides without loss
of activity or selectivity (Table 8.6) [50]. According to
1
H NMR, the zwitterionic
form of flavin 26 predominates, which explains why 26 stays in the ionic liquid after
the extraction of the product.
Heterogeneous catalytic oxidation of sulfides in ionic liquids by anhydrous H
2
O
2
or urea hydroperoxide with MCM-41 and related mesoporous catalysts containing Ti
or Ti and Ge was studied by Hardacre and coworkers [51]. The Ti-based catalyst gave a
quite selective sulfoxidation. Addition of Ge to Ti increased the rate of the oxidation
but reduced the selectivity toward sulfoxide [51a].
Chiral flavins have been used to obtain an asymmetric sulfoxidation with H
2
O
2
as
the oxidant [45a, 52]. Flavin 27, with planar chirality, was used to oxidize different aryl
methyl sulfides with 35% H
2
O
2
. The hydrogen peroxide was added slowly over 5 days
at 20
C to the substrate and the catalyst. The best result was obtained with the
p-tolyl derivative, which gave 65% ee (Eq. (8.12)).
8.2 Oxidation of Sulfides to Sulfoxides
j
291
Table 8.6 Recycling of the flavin-catalyst in ionic liquid.
a),b)
.
R
S
R'
Heq.~1.5
2
O
2
MeOH,
26/ [BMIm]PF
6
R
S
R'
O
liquidionicinCatalyst
recycledis
Entry Sulfide Yield of sulfoxide
c)
(%)
Run 1 Run 2 Run 3 Run 4 Run 5 Run 6/7
1
p
-Me-C
6
H
4
S
78 84 80 88 91 89/-
2
d)
p
-MeO-C
6
H
4
S
87
e)
87
e)
87
e)
87
e)
87
e)
87
e)
/88
3
p
-Me-C
6
H
4
S
Bu
95 98 89 86 92 94/-
4
S
91 94 88 ——-/-
a) Unless otherwise noted: The reactions were run using 1 mmol of sulfide in 0.5 mL [BMIm]P F
6
and 3.2 mL MeOH. Flavin 26 (6.3 mg, 2 mol%) and H
2
O
2
(150–170 mL, 1.5 equiv.) were added
and reaction stirred for 1.5–2.5 h.
b) Conversion determined by
1
H-NMR and was 96– 99%.
c) Isolated yield.
d) A seventh run gave 88% yield.
e) The crude products of runs 1–6 were combined to give 522% isolated yield converted on one run,
which gives in average 87% yield per run.
p
-MeC
6
H
4
S
Me
p-MeC
6
H
4
S
flavin 24 (cat.)
H
2
O
2
MeOH-H
2
O
-20
o
C,5days
O
*
N
N
N
N
O
(CH
2
)
8
O
OEt
27
+
ClO
4
-
65%ee
ð8:12Þ
292
j
8 Selective Oxidation of Amines and Sulfides
