Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

CO
2
H
CO
2
H
RuCl
3
(cat.)
NaOCl
CCl
4
-H
2
O
86%
ð7:5Þ
OH
OH
RuCl
3
(cat.)
NaIO
4
EtOAc-H
2
O-CH
3
CN
58%
0.5 min, 0
o
C
ð7:6Þ
RuCl
3
(cat.)
NaIO
4
0
o
C
EtOAc-CH
3
CN
O
OHOH
85%
ð7:7Þ
Octavalent RuO
4
generated from an RuCl
3
/hypochlorite or periodate system is
usually too reactive, and the C¼C bond cleavage is often a major reaction; however,
the addition of a bipyridine ligand facilitates the epoxidation of alkenes, because it
works as an electron-donating ligand to enhance the electron density on the metal and
to modulate the reactivity of RuO
4
[17–20]. RuCl
3
associated with bipyridyl [17] and
phenanthroline [18] catalyzes the epoxidation of alkenes with sodium periodate
(Eq. (7.8)). The reactions are stereospecific for both cis- and trans-alkenes. The dioxo-
ruthenium(IV) complex [RuO
2
(bpy){IO
3
(OH)
3
}]1.5H
2
O(2) was isolated by the
reaction of RuO
4
with bipyridyl in the presence of NaIO
4
, and the complex acts as
an efficient epoxidation catalyst under similar conditions (Eq. (7.8)) [19]. Ketohy-
droxylation of alkenes can be carried out by RuCl
3
-catalyzed oxidation with oxone
under buffered conditions [21].
Ph
Ph
Ph
Ph
O
NN
O
Ru
O
O
O
I
OH
OH
OH
O
N
N
RuCl
3
•nH
2
O (cat.)
L =
L, NaIO
4
CH
2
Cl
2
–H
2
O
90%
2
Ru cat. =
99%
ð7:8Þ
1,2-Dihaloalkenes are oxidized to a-diketones in a variety of norbornyl derivatives,
which can serve as highly potent and inextricable templates for strained polycyclic
unnatural compounds (Eq. (7.9)) [22].
7.2 RuO
4
-Promoted Oxidation
j
243

OMeMeO
Cl
OAc
Cl
Cl
Cl
OMeMeO
Cl
OAc
O
O
Cl
RuCl
3
•nH
2
O (cat.)
NaIO
4
99%
CH
3
CN–H
2
O
0
o
C
ð7:9Þ
Aromatic and furan rings are smoothly converted into carboxylic acids (Eqs. (7.10)
and (7.11)) [23]. Terminal alkynes undergo a similar oxidative cleavage to
afford carboxylic acids, while internal alkynes are converted into diketones
(Eq. (7.12)) [24, 25].
O
Ph
H
H
O
MeO
2
C
H
H
RuCl
3
(cat.)
NaIO
4
CCl
4
-H
2
O-CH
3
CN
CH
2
N
2
1)
2)
85%
ð7:10Þ
Ph
OCOPh
HO
2
C
OCOPh
RuCl
3
(cat.)
HIO
4
CCl
4
-H
2
O-CH
3
CN
80%
ð7:11Þ
TBDMSC
13
H
27
C
13
H
27
TBDMS
O
O
RuO
2
(cat.)
NaIO
4
CCl
4
-H
2
O-CH
3
CN
95%
ð7:12Þ
The oxidation of allenes gives a,a-dihydroxyketones (Eq. (7.13)) [26]. Various
heteroatom-containing compounds undergo oxidation of methylene groups at the
a-position. Ethers are converted into esters and lactones [27]. The efficiency of the
a-oxidation of ethers can be improved by pH control using hypochlorite in biphasic
media (Eq. (7.14)) [27a]. Tertiary amines [28] and amides [29] undergo similar
oxygenation reactions at the a-position of nitrogen to afford the corresponding
amides and imides, respectively (Eq. (7.15)). Carbon–carbon side-chain fragmenta-
tion occurs when N,C-protected serine and threonine are subjected to oxidation. The
method has been successfully applied to the NC bond scission of peptides in serine
or threonine residue (Eq. (7.16)) [30].
CH
3
t
-Bu
CO
2
Et
CH
3
CO
2
Et
OHOH
O
t
-Bu
72%
RuCl
2
(PPh
3
)
3
(cat.)
NaIO
4
EtOAc-H
2
O-CH
3
CN
ð7:13Þ
C
3
H
7
OC
4
H
9
C
3
H
7
OC
4
H
9
O
RuCl
2
(dppp)
2
(cat.)
NaOCl
CH
2
Cl
2
-H
2
O (pH 9.5)
93%
ð7:14Þ
244
j
7 Ruthenium-Catalyzed Oxidation for Organic Synthesis

N
Boc
OSiMe
2
-
t
-Bu
RuO
2
(cat.)
NaIO
4
CCl
4
-H
2
O
N
Boc
OSiMe
2
-
t
-Bu
90%
O
ð7:15Þ
Boc-Ala-Ala-Ser-OMe
RuCl
3
(cat.)
NaIO
4
pH 3 phosphate buffer
CCl
4
-H
2
O-CH
3
CN
Boc-Ala-Ala-NH
2
78%
ð7:16Þ
Unactivated alkanes can also be oxidized with the RuCl
3
/NaIO
4
system. Tertiary
carbon–hydrogen bonds undergo chemoselective hydroxylation to afford the corre-
sponding tertiary alcohols (Eq. (7.17)) [31].
H
RuCl
3
(cat.)
NaIO
4
CCl
4
-H
2
O-CH
3
CN
OH
69%
ð7:17Þ
Bridgehead carbons of adamantane [32], pinane [33], and fused norbornane [34]
undergo selective hydroxylation under similar reaction conditions. Methylene
groups of cycloalkanes undergo hydroxylation and then subsequent oxidation to
afford the corresponding ketones [35]. In general, methyl groups of alkanes undergo
no reaction with RuO
4
, while the methyl group of toluene can be converted into the
corresponding carboxylic acid (Eq. (7.18)) [36].
CH
3
CO
2
H
RuCl
3
(cat.)
NaOCl
ClCH
2
CH
2
Cl-H
2
O
Bu
4
N
+
Br
-
92%
ð7:18Þ
7.3
Oxidation with Low-Valent Ruthenium Catalysts and Oxidants
The treatment of a low-valent ruthenium catalysts with an oxidant generates middle-
valent Ru ¼ O species, which often show a different reactivity from that of the RuO
4
oxidation.
7.3.1
Oxidation of Alkenes
The epoxidation of alkenes with metalloporphyrins has been studied as model
reactions of cytochrome P-450 [37]. Ruthenium porphyrins such as Ru(OEP)
(PPh
3
)Br (OEP ¼ octaethylporphyrinato) have been examined for the catalytic
oxidation of styrene with PhIO [38]. Hirobe and coworkers [39] and Groves and
7.3 Oxidation with Low-Valent Ruthenium Catalysts and Oxidants
j
245
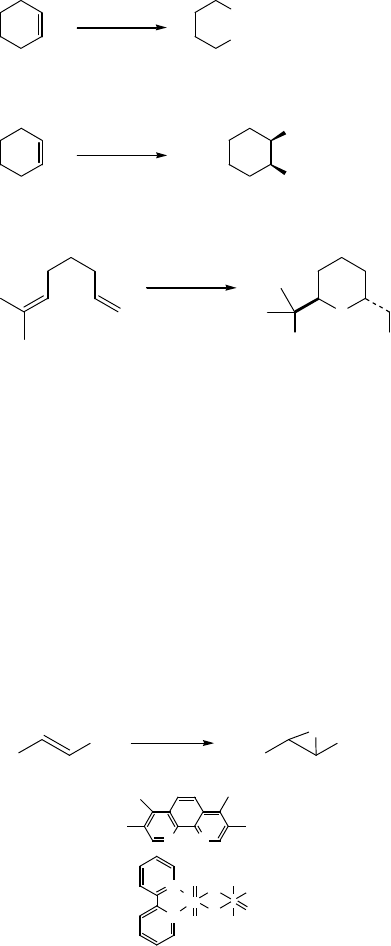
coworkers [40] reported that the ruthenium porphyrin-catalyzed oxidations of
alkenes with 2,6-dichloropyridine N-oxide gave the corresponding epoxides in
high yields (Eqs. (7.19) and (7.20)). The chlorine substituents at the 2- and 6-
positions on pyridine N-oxide are necessary for high efficiency, because simple
pyridine coordinates to the ruthenium more strongly and retards the catalytic
activity. Ru(TMP)Cl
2
-catalyzed oxidation of terminal alkenes with 2,6-dichloro-
pyridine N-oxide gave a Wacker-type product of an aldehyde via epoxidation-
isomerization mechanism (TMP ¼ tetramethylporphyrinato) [41a]. Now, rutheni-
um porphyrin-catalyzed aerobic oxidation of terminal aryl alkenes to aldehyde can
be carried out ef ficiently [41b]. Thus, the reaction of styrenes in the presence of
Ru(TMP)Cl
2
(2 mol%), NaHCO
3
, and H
2
O in CDCl
3
gives the corresponding
aldehydes by tandem epoxidation-isomerization. Nitrous oxide (N
2
O) can also be
used as an oxidant for the epoxidation of trisubstituted alkenes in the presence of
ruthenium porphyrin catalyst [42]. Importantly, oxidative cleavage of alkenes to give
dicarboxylic acid and cis-dihydroxylation can be carried out by either [Ru(Me
3
tacn)
(CF
3
CO
2
)
2
(OH
2
)]
þ
/H
2
O
2
or [Ru(Me
3
tacn)Cl
3
]/H
2
O
2
[43]. Terminal alkenes under-
go selective CC bond cleavage to give aldehydes with cis-Ru(dmp)(H
2
O)
2
/
H
2
O
2
[44].
O
C
6
H
6
,30°C
100%
Ru(TMP)(O)
2
(3) (cat.)
2,6-dichloropyridine
N
-oxide
ð7:19Þ
O
CH
2
Cl
2
, 65°C
Ru(TPFPP)(CO) (
4) (cat.)
NN
N
N
Ru
O
O
2,6-dichloropyridine
N
-oxide
96%
Ru(TPFPP)(CO) (
4)
Ru(TMP)(O)
2
(3)
NN
N
N
Ru
C
F
F
FF
F
F
FF
F
F
F
F
F
F
F
F
FF
F
F
O
TPFPP: tetrakis(pentafluorophenyl)porphyrinatoTMP: tetramesitylporphyrinato
ð7:20Þ
Non-porphyrin ruthenium complexes such as [RuCl(DPPP)
2
] (DPPP ¼ 1,3-bis
(diphenylphosphino)propane), [Ru(6,6-Cl
2
bpy)
2
(H
2
O)
2
], binaphthyl-ruthenium
complex, and RuCl
3
catalyze oxidations of alkenes with PhIO [45], t-BuOOH [46],
PhI(OAc)
2
[47], or H
2
O
2
[48] to give the corresponding epoxides in moderate
yields.
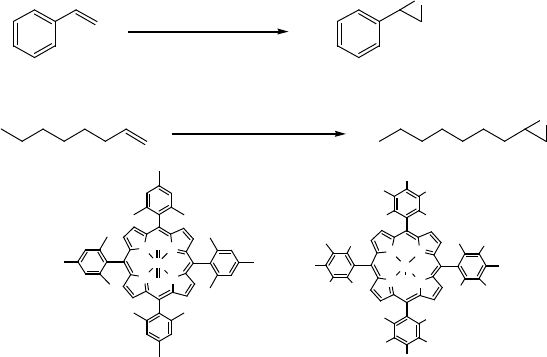
The ruthenium-catalyzed aerobic oxidation of alkenes has been explored by several
groups. Groves and coworkers reported that Ru(TMP)(O)
2
(3)-catalyzed aerobic
epoxidation of alkenes proceeds under 1 atm of molecular oxygen without any
246
j
7 Ruthenium-Catalyzed Oxidation for Organic Synthesis
reducing agent [40b]. A ruthenium-containing polyoxometalate, {[WZnRu
2
(OH)
(H
2
O)](ZnW
9
O
34
)
2
}
11
[49], and a sterically hindered ruthenium complex,
[Ru(dmp)
2
(CH
3
CN)
2
](PF
6
) (dmp ¼ 2,9-dimethyl-1,10-phenanthroline) [50], are also
effective for epoxidation with molecular oxygen. Knochel and coworkers reported
that the ruthenium catalyst bearing perfluorinated 1,3-diketone ligands catalyzes the
aerobic epoxidation of alkenes in a perfluorinated solvent in the presence of
i-C
3
H
7
CHO [51]. Asymmetric epoxidations have been reported using ruthenium
complexes with oxidants such as PhIO [52], PhI(OAc)
2
[53], 2,6-dichloropyridine
N-oxide [54–56], hydrogen peroxide [57], and molecular oxygen [58].
It was postulated that one of the possible intermediates for metalloporphyrin-
promoted epoxidation is the intermediate 5 (Scheme 7.2) [59].
If one could trap the intermediate 5 with external nucleophiles, such as water,
a new type of catalytic oxidation of alkenes could be performed. Indeed,
a transformation of alkenes into a-ketols was discovered to proceed highly
efficiently. Thus, the low-valent ruthenium-catalyzed oxidation of alkenes with
peracetic acid in an aqueous solution under mild conditions gives the correspond-
ing a-ketols, which are important key structures of various biologically active
compounds (Eq. (7.21)) [60].
R
2
R
3
R
1
R
2
R
3
R
1
O
HO
CH
3
CO
3
H
Ru cat.
CH
2
Cl
2
-CH
3
CN-H
2
O
ð7:21Þ
Typically, the RuCl
3
-catalyzed oxidation of 3-acetoxy-1-cyclohexene (6) with per-
acetic acid in H
2
O-CH
3
CN-CH
2
Cl
2
(1 : 1 : 1) gave (2R
,3S
)-3-acetoxy-2-hydroxycy-
clohexanone (7) chemo- and stereoselectively in 78% yield (Eq. (7.22)).
OAc
O
OH
OAc
CH
3
CO
3
H
rt
7
CH
2
Cl
2
-CH
3
CN-H
2
O
6
RuCl
3
•nH
2
O (cat.)
ð7:22Þ
The oxidation is highly efficient and quite different from that promoted by RuO
4
.
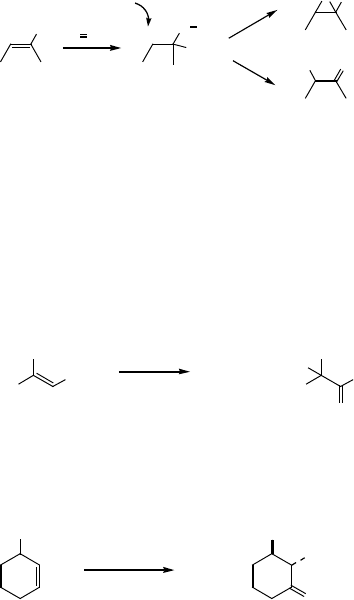
Indeed, the oxidation of 1-methylcyclohexane (8) under the same conditions gives 2-
hydroxy-2-methylcyclohexanone (9) (67%), while the oxidation of the same substrate 8
under conditions in which the RuO
4
is generated catalytically gives 6-oxoheptanoic
acid (10) (91%) (Eq. (7.23)).
H
OM
MO
H
H
O
O
Nu
+
Nu
– M
5
– MH
Scheme 7.2
7.3 Oxidation with Low-Valent Ruthenium Catalysts and Oxidants
j
247
O
OH
CO
2
H
O
NaIO
4
CCl
4
-CH
3
CN-H
2
O
RuCl
3
•nH
2
O (cat.)
10
CH
3
CO
3
H
RuCl
3
•nH
2
O (cat.)
CH
2
Cl
2
-CH
3
CN-H
2
O
9
8
ð7:23Þ
This particular oxidation can be applied to the oxidation of substituted alkenes
having functional groups such as acetoxy, methoxycarbonyl, and azide groups to give
the corresponding a-ketols in good to excellent yields. The oxidation of 3-azide-1-
cyclohexene (11) gave (2S
,3R
)-3-azide-2-hydroxycyclohexanone (12) chemo- and
stereoselectively (65%) (Eq. (7.24)).
N
3
O
OH
N
3
1211
CH
3
CO
3
H
RuCl
3
•nH
2
O (cat.)
CH
2
Cl
2
-CH
3
CN-H
2
O
ð7:24Þ
The efficiency of the present reaction has been demonstrated by the synthesis of
cortisone acetate 15 [60], which is a valuable anti-inflammatory agent. The oxidation
of 3b, 21-diacetoxy-5a-pregn-17-ene (13) proceeds stereoselectively to give 20-oxo-5a-
pregnane-3b,17a,21-triol 3,21-diacetate (14) (57%) (Eq. (7.25)). Conventional treat-
ment of 14 followed by microbial oxidation with Rhizopus nigricaus gave cortisone
acetate (15).
OAc
OAc
O
OH
AcO
AcO
OAc
O
OH
O
O
RuCl
3
(cat.)
CH
3
CO
3
H
CH
3
CN-
CH
2
Cl
2
-H
2
O
13
14
Cortison acetate (15)
ð7:25Þ
Furthermore, the method can be applied to the synthesis of 4-demethoxyadria-
mycinone, which is the side-chain of cancer drug adriamycins such as idarubicin and
annamycin (16). The ruthenium-catalyzed oxidation of allyl acetate 17 gives the
corresponding a-hydroxyketone 18 in 60% yield (Eq. (7.26)) [61]. The reaction was
248
j
7 Ruthenium-Catalyzed Oxidation for Organic Synthesis
also applied to the oxidation of a ,b-unsaturated carbonyl compounds 19, and this
provides a new method for the synthesis of a-oxo-ene-diols 20 (Eq. (7.27)) [62].
O
OAcO
OH
O-
t
-Bu
OAc
O
OAcO
OH
O-
t
-Bu
OAc
O
OH
O
OHO
OH
O
O
OH
OH
O
I
Me
OH
OH
RuCl
3
(cat.)
CH
3
CO
3
H
CH
3
CN-
CH
2
Cl
2
-H
2
O
18
annamycin
17
16
ð7:26Þ
O
i
-Pr
O
i
-Pr
HO
HO
CH
3
CO
3
H
CH
2
Cl
2
-CH
3
CN-H
2
O
RuCl
3
•nH
2
O (cat.)
55%
2019
0 to 20°C
ð7:27Þ
7.3.2
Oxidation of Alcohols
The ruthenium-catalyzed oxidation of alcohols has been reported using various
catalytic systems, which include RuCl
2
(PPh
3
)
3
catalyst with oxidants such as N-
methylmorpholine N-oxide (NMO) [63], iodosylbenzene [64], TMSOOTMS [65],
RuCl
3
with hydrogen peroxide [66], K
2
RuO
4
with peroxodisulfate [67], and Ru(py-
box)(Pydic) complex with diacetoxyiodosylbenzene [68]. The salt of the perruthenate
ion with a quaternary ammonium salt, (n-Pr
4
N)(RuO
4
) (TPAP), which is soluble in
a variety of organic solvents, shows far milder oxidizing properties than those of
RuO
4
[69]. One of the key features of the TPAP system is its ability to tolerate other
potentially reactive groups. For example, double bonds, polyenes, enones, halides,
cyclopropanes, epoxides, and acetals all remain intact during TPAP oxidation [69].
The oxidation of primary and secondary alcohols with TPAP gives the corresponding
aldehydes and ketones (Eqs. (7.28) and (7.29)), whereas oxidation with RuO
4
results
in the formation of carboxylic acid. NaOCl can also be used as an oxidant for the
TPAP-catalyzed oxidation of secondary alcohols [70].
7.3 Oxidation with Low-Valent Ruthenium Catalysts and Oxidants
j
249
OTBDMS
OH
O
OTBDMS
O
O
(
n
-Pr
4
N)(RuO
4
)
(cat.)
NMO
MS4A
CH
2
Cl
2
70%
2221
ð7:28Þ
O
H
H
TBDMSO
OH
OTBDMS
O
H
H
TBDMSO
OTBDMS
O
97%
2423
ð7:29Þ
The RuCl
2
(PPh
3
)
3
-catalyzed reaction of secondary alcohols with t-BuOOH gives
ketones under mild conditions [71, 72]. This oxidation can be applied to the
transformation of cyanohydrins into acyl cyanides [71], which are excellent acylating
reagents. Typically, the oxidation of cyanohydrin 25 with two equiv. of t-BuOOH in dry
benzene at room temperature gives benzoyl cyanide (26) in 92% yield (Eq. (7.30)). It is
worth noting that the acyl cyanides thus obtained are excellent reagent for the
chemoselective acylation reaction. The reaction of amino alcohols with acyl cyanides
gives N-acylated amino alcohols selectively. Furthermore, primary amines are
selectively acylated in the presence of secondary amines [73]. The utility of the
reaction has been illustrated by the short-step synthesis of maytenine (27) (Eq. (7.30)).
A ruthenium complex [Cn
Ru(CF
3
CO
2
)
3
(H
2
O)] (Cn
¼ N,N
0
,N
00
-trimethyl-1,4,7-tria-
zacyclononane) catalyst can be used for the oxidation of alcohols with t-BuOOH [74].
CNPh
OH
CNPh
O
NPh
H
O
N
H
H
PhN
H
2
NN
H
NH
2
RuCl
2
(PPh
3
)
3
C
6
H
6
, rt
t
-BuOOH
2625
(cat.)
maytenine (27) 92%
92%
CH
2
Cl
2
- HCN
O
ð7:30Þ
Various aliphatic and aromatic secondary alcohols can be oxidized with peracetic
acid in the presence of RuCl
3
catalyst to give the corresponding ketones highly
efficiently [75].
The generation of peracetic acid in situ provides an efficient method for the aerobic
oxidation of alcohols. The oxidation of various aliphatic and aromatic alcohols can be
carried out at room temperature with molecular oxygen (1 atm) in the presence of
acetaldehyde and RuCl
3
–Co(OAc)
2
bimetallic catalyst [76]. This method is highly
convenient, because the products can be readily isolated simply by removal of both
acetic acid and the catalyst by washing with a small amount of water.
An alternative method for the oxidation of alcohols is dehydrogenative oxidation
via a hydrogen transfer reaction [2a]. The process of dehydrogenation of alcohols by
250
j
7 Ruthenium-Catalyzed Oxidation for Organic Synthesis
metal catalysts is of importance from biological and industrial viewpoints. Alcohols
can be activated efficiently with low-valent ruthenium complexes to give the carbonyl
dihydridoruthenium intermediates (Eq. (7.31)). Capture of the intermediates with
nucleophiles provides various catalytic oxidative condensations of alcohols. Thus,
primary alcohols undergo oxidative condensation upon treatment with RuH
2
(PPh
3
)
4
[77] or Ru(CO)
3
(g
4
-tetracyclone) [78] catalyst to give esters along with evolution of
molecular hydrogen (Eq. (7.32)). The RuH
2
(PPh
3
)
4
-catalyzed reaction of 1,n-diol
(n „ 4,5) gives the corresponding polyesters and molecular hydrogen [2a]. Similar
treatment of diols affords the corresponding lactones. In the presence of hydrogen
acceptor the RuH
2
(PPh
3
)
4
-catalyzed oxidative condensation of alcohols to esters
proceeds at low temperature (Eq. (7.33)) [77]. As a hydrogen acceptor,
a,b-unsaturated ketones and other substrates have been used; however, acetone
was found to be the most effective and convenient hydrogen acceptor [77b–f]. The
oxidative cross-esterification of alcohols with methanol is performed in the presence
of Ru(PPh
3
)
3
(CO)H
2
catalyst (Eq. (7.34)) [79a]. Application of the present reaction
provides novel catalytic reactions. Thus, the oxidative condensations of aldehydes
with alcohols to esters and of aldehydes with water give acids or esters [2a,77b].
Hartwig and Milstein reported that dehydrogenative cyclization of diols to lactones
can be carried out using Ru(PMe
3
)
2
Cl
2
(eda) (eda: ethylenediamine) [79b] and RuHCl
(PNN)(CO) (PNN: 2-(di-t-butylphosphinomethyl)-6-(diethylaminomethyl)pyridine)
catalyst [79c]. Direct conversion of alcohols to acetals and H
2
catalyzed by an
acridine-based ruthenium pincer complex is performed efficiently (Eq. (7.35)) [80].
R
1
R
2
CHOH
Ru
R
1
R
2
C=O
H-Ru-H
Nu
ð7:31Þ
2 RCH
2
OH
RCO
2
CH
2
2 HR
2
Ru(CO)
3
O
Ph
Ph
Ph
Ph
(cat.)
RuH
2
(PPh
3
)
4
(cat.)
+
or
ð7:32Þ
RuH
2
(PPh
3
)
3
(cat.)
OH
OH
O
O
acetone (hydrogen acceptor)
toluene
98%
180 °C, 3 h
ð7:33Þ
Ru(PPh
3
)
2
(CO)H
2
(cat.)
xamphos
toluene,
83%
110 °C
Ph
OH
COPh
2
Me
crotonitrile (hydrogen acceptor)
MeOH+
ð7:34Þ
7.3 Oxidation with Low-Valent Ruthenium Catalysts and Oxidants
j
251

(cat.)
conv. 92%
157 °C
R
OH
N
Ru
P
P
H
Cl
CO
i
-Pr
i
-Pr
i
-Pr
i
-Pr
R
O
O
R
R
+ H
2
+ H
2
O
ð7:35Þ
Direct methods for the synthesis of secondary amines from alcohols and amines
using ruthenium catalysts were discovered in 1981–1982 [81a,b], although Pd
catalyst [81c] and RhH(PPh
3
)
4
[81d] are excellent catalysts for activation of alcohols
in the presence of amines. The RuH
2
(PPh
3
)
4
catalyst is an excellent general catalyst
for the activation of alcohols in the presence of amines, and can be used for the
synthesis of aliphatic amines [81a]. On the other hand RuCl
2
(PPh
3
)
3
is a more
reactive catalyst, but this catalyst can be used for synthesis of aryl amines [81b].
Recently, Beller reported that the catalytic system of Ru
3
(CO)
12
and N-phenyl-2-
(dicyclohexylphosphanyl)pyrrole can be used for amination of alcohols [81e]. These
methods are now well documented as borrowing hydrogen [2a] (see excellent
review in Ref.[81f]).
The first example of the ruthenium-catalyzed synthesis of amides from alcohols
and amines was reported by Murahashi et al. in 1991 [82a]. The contrast results
were obtained from the RuH
2
(PPh
3
)
4
-catalyzed reaction of 5-aminopentanol. Thus,
piperidine was obtained in 79% yield, while similar treatment in the presence of
a hydrogen acceptor of 1-phenyl-1-buten-3-one gave piperidone in 65% yield
(Eq. (7.36)). Recently, Williams reported the intermolecular amidation reaction of
benzyl alcohols with amines in the presence of [Ru(p-cymene)Cl
2
]
2
and 3-methyl-2-
butanone [82b].
Milstein et al. discovered an extremely important result, that is, a ruthenium
complex with a PNN ligand, where PNN is 2-(di-t-butylphosphinomethyl)-6-(diethy-
laminomethyl)pyridine, promotes the direct dehydrogenative acylation of amines
with alcohols catalytically with liberation of H
2
(Eq. (7.37)) [83].
H
2
OHN
N
H
N
H
O
RuH
2
(PPh
3
)
4
RuH
2
(PPh
3
)
4
PhCH=CHCOCH
3
79%
65%
ð7:36Þ
252
j
7 Ruthenium-Catalyzed Oxidation for Organic Synthesis
