Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.


162
Chapter
7
one or more of these emission lines are filtered by an optical filter or spectrome-
ter. The intensity of the line
is
a measure of the concentration of the selected
atom.
7.3
HISTORICAL DEVELOPMENT
OF
THE
PLASMA DETECTOR
Broida and Morgan
[
13
were the first, in 1952, to describe a system for the
analysis of gaseous mixtures of hydrogen, deuterium and air based on optical
emission spectroscopy with photoelectric detection. An electrodeless discharge
at
150 MHz
in
a continuous flow gas system was used in conjunction with a
high-resolution grating monochromator. Each component could be determined
with an accuracy better than
0.1
%
of the total mixture. Gas chromatography was
hardly in use at that time, and it was not until
1965
that McCormack, et al. [2]
first reported a system in which the effluent of a gas chromatographic column
was fed into an argon microwave discharge of 2450
MHz
at atmospheric pres-
sure. The emission was detected
in
the W-visible region. Under the conditions
described by these authors the eluted components were only partly fragmented in
the plasma and the emitted spectrum consisted of both atomic and molecular
spectra. They found very low selectivity ratios relative to n-hexane (between
10
and
loo),
the sensitivity being strongly dependent on the compound involved.
Bache and Lisk [3-61 were able to detect selectively the atomic lines of halogens,
phosphorus and sulphur: they used a low-pressure microwave-induced plasma of
helium providing nearly complete atomization
of
organic compounds. However,
reproducible operation was impeded by the deposition
of
carbon on the wall of
the quartz tube in which the plasma was confined.
Moye
[7]
used an almost similar system for the detection of phosphorus and
halogen compounds. He used argon-helium gas mixtures
and
found lower limits
of detection, between
0.1 and
1
ng. He examined a few experimental parameters,
such as the quartz tube diameter. The
GC
column was operated under reduced
pressure and the pressure in the plasma was maintained at
30
mbar. Experimen-
tal work was also done with
DC
plasmas.
In
1968
Braman and
Dynako
[8] used a
DC
discharge
in
helium together with a GC column. These authors used interfer-
ence filters or a spectrometer to yield limits
of
detection in the picogram-per-
second range for elements such as
F,
C1,
Br and
I.
The maximum amount of car-
bon that could enter the detector was
0.1
mg with an optimum power supplied to
the plasma
of
10
W.
Dagnall [9] used a microwave system and found that the
carbon deposits on the walls of the quartz tube could be burned off by operating
the plasma with air. However, this led to degradation and the need for replace-
ment of the quartz tube. Braun et al.[
101
removed the carbon by addition of
0.5
-
5%
O2
to the helium carrier gas. This procedure allowed the determination of
C

Microwave plasma detectors
163
and
N
through atomic emission lines in the vacuum
UV
region. In
1972
these
authors tested a DC-based plasma detection system in combination with a gas
chromatograph. At high sensitivities the metal electrodes started to evaporate,
which made the quartz tube less bright and impeded long term stability. Moreo-
ver, the electrodes reacted with halogens.
With a microwave plasma McLean et al.
[
1
11
reduced the oxygen concentra-
tion to
0.1
-
1
%
and used spectral lines in the visible region for the selective de-
tection of halogens, H, D, C and
N.
They also found that nitrogen could act as a
carbon scavenger and this discovery enabled oxygen to be included in the range
of detectable elements. A commercially available system based on this publica-
tion was produced by Applied Research Laboratories in England. This system
used a low-pressure plasma, generated within an Evenson
214L
resonance cav-
ity. The minimum detectable levels (MDL) were about
100
pg/s. The selectivity
of the elements relative to carbon was only about
100.
Another possibility to improve the MDLs of the low-pressure plasma, was by
increasing the plasma pressure. With the Evenson
214L
cavity, used to create the
plasma, the power reflected to the microwave generator increased with the pres-
sure in the quartz tube. With this cavity it was not possible to operate at an at-
mospheric pressure. Beenakker
[12,13]
described a microwave cavity that is able
to work with He at atmospheric pressure. He reported lower limits of detection
between
1
and
100
pg/s. Using this type of cavity, Quimby et al.
[14]
in
1978,
measured limits around
10
pg/s. At that time it was not yet clear which design
was best to create a plasma. The same group
[15]
reported the use of a DC
plasma, using Ar as a carrier for the determination of metals. Moisan
[16],
and
later Abdallah
[17],
described a Surfatron to create a stable plasma. Our own
experiments with all four types gave rise to the following findings: the Surfatron
appeared to be very difficult to operate and high MDLs were obtained. The DC
or AC plasma did not produce sufficiently low limits of detection for non-metals.
We furthermore encountered problems with the electrodes (reaction and glow-
ing) at high power. Both the low-pressure plasma with the Evenson cavity and
the atmospheric plasma of Beenakker produced lower limits
of
detection, be-
tween
0.1
and
10
pg/s. For the Beenakker cavity this was also reported by Estes
et al.
[18,19].
However the selectivity relative to C impeded the use
of
these
systems.
In order to improve this ratio (about
loo),
Applied Research Laboratories in
the first commercially available instrument, made an improvement by subtract-
ing a fraction
of
the carbon signal from the measured line (e.g. chlorine). This
improved the selectivity ratio relative to carbon to about
1000.
However, the cor-
rection had to be adjusted
for
every emission line and moreover, the correction
was not always proportional to the carbon concentration and could only be ap-
plied over a small concentration range. Wavelength modulation with a refractor
References
p.
200

164
Chapter
7
plate in the spectrometer allowed correction via nearby wavelengths on either
side of the spectral line. When a system requires short response times, e.g. for
capillary columns, the high frequencies necessary cause a decrease in signal-to-
noise ratio. The resulting loss in limit of detection compared to non-modulating
systems was demonstrated by Koirtyohann et al. [20]. The sectored wheel sys-
tem as described by McCaffrey [21] had a better signal-to-noise ratio, because
this system wasted less time travelling from the element line
to the background.
However, the observation time
on
the element emission line is maximally
50%
of the overall measuring time, while noise is measured continuously. De Wit et
al. 1221 developed a triple-slit-exit system. This construction permitted continu-
ous and simultaneous measurement
of
the spectral line intensity and the back-
ground and could be used up to very short system time constants without loss
in
lower limit of detection. In the next part of this chapter we will discuss a
number of parameters influencing the performance of both the low-pressure and
the atmospheric pressure plasma together with a triple-slit exit system. In
1990
Quimby [23,24] described an atomic emission detector (AED) which combined
the good lower limits of detection of the atmospheric helium plasma with
the good selectivity obtainable with diode array detection. The performance of
this system, together with some applications, will be described in sections
6
and
7.
7.4 DESCRIPTION
AND
EVALUATION
OF
A HOME-BUILT ATOMIC
EMISSION DETECTOR
This section contains a description and evaluation of the
two
types of micro-
wave plasma detectors used, i.e. the low-pressure plasma (LPP) and the atmos-
pheric-pressure plasma (APP) detector.
A
few parameters that influence the minimum and upper detectable level
(MDL and
UDL)
and the selectivity will be briefly discussed. A schematic dia-
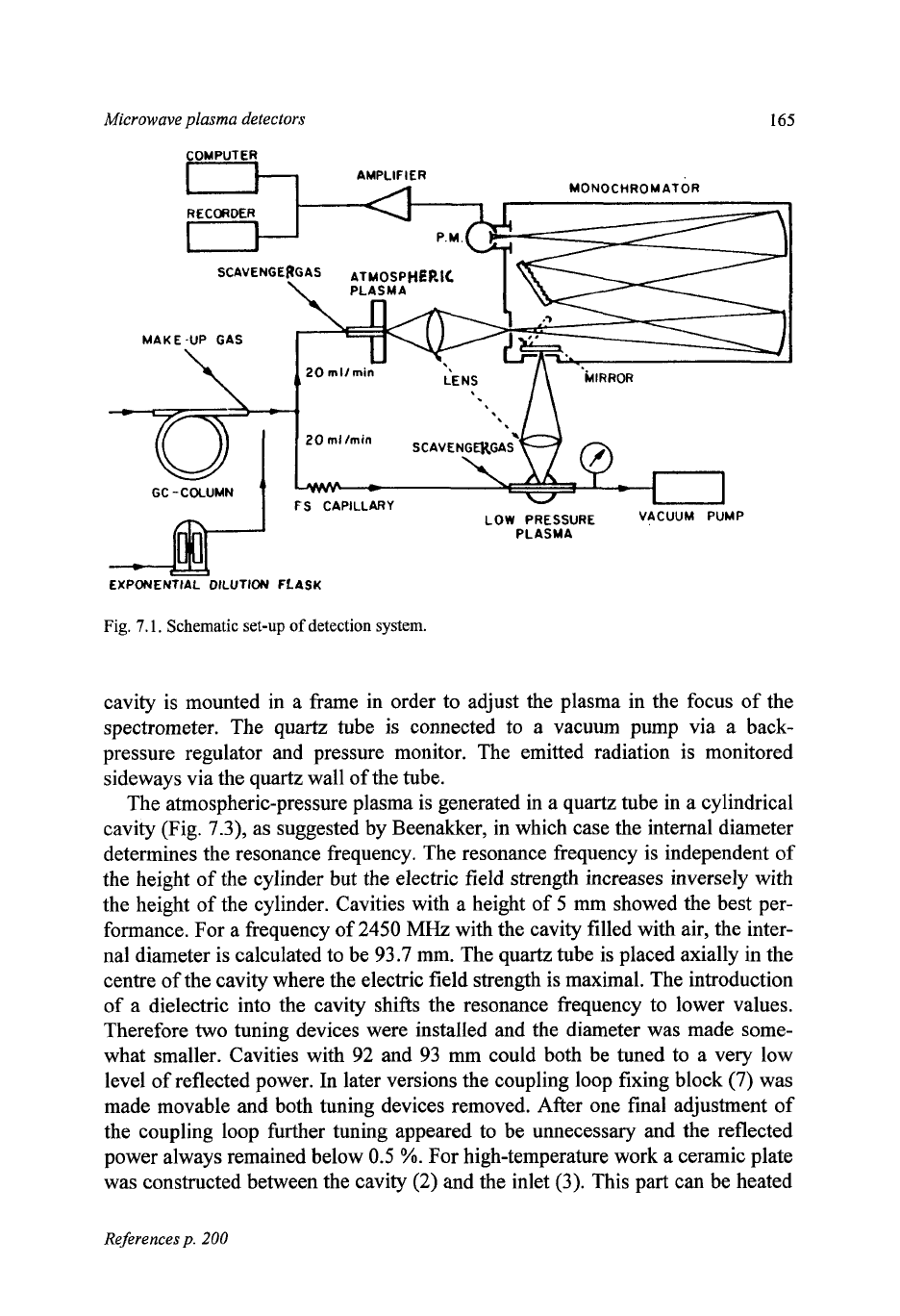
gram of the set-up for both plasmas is given in Fig. 7.1. Further details are also
given
in
Table 7.1.
7.4.1 Description
of
the apparatus
7.4.
I.
1
Microwave cavities
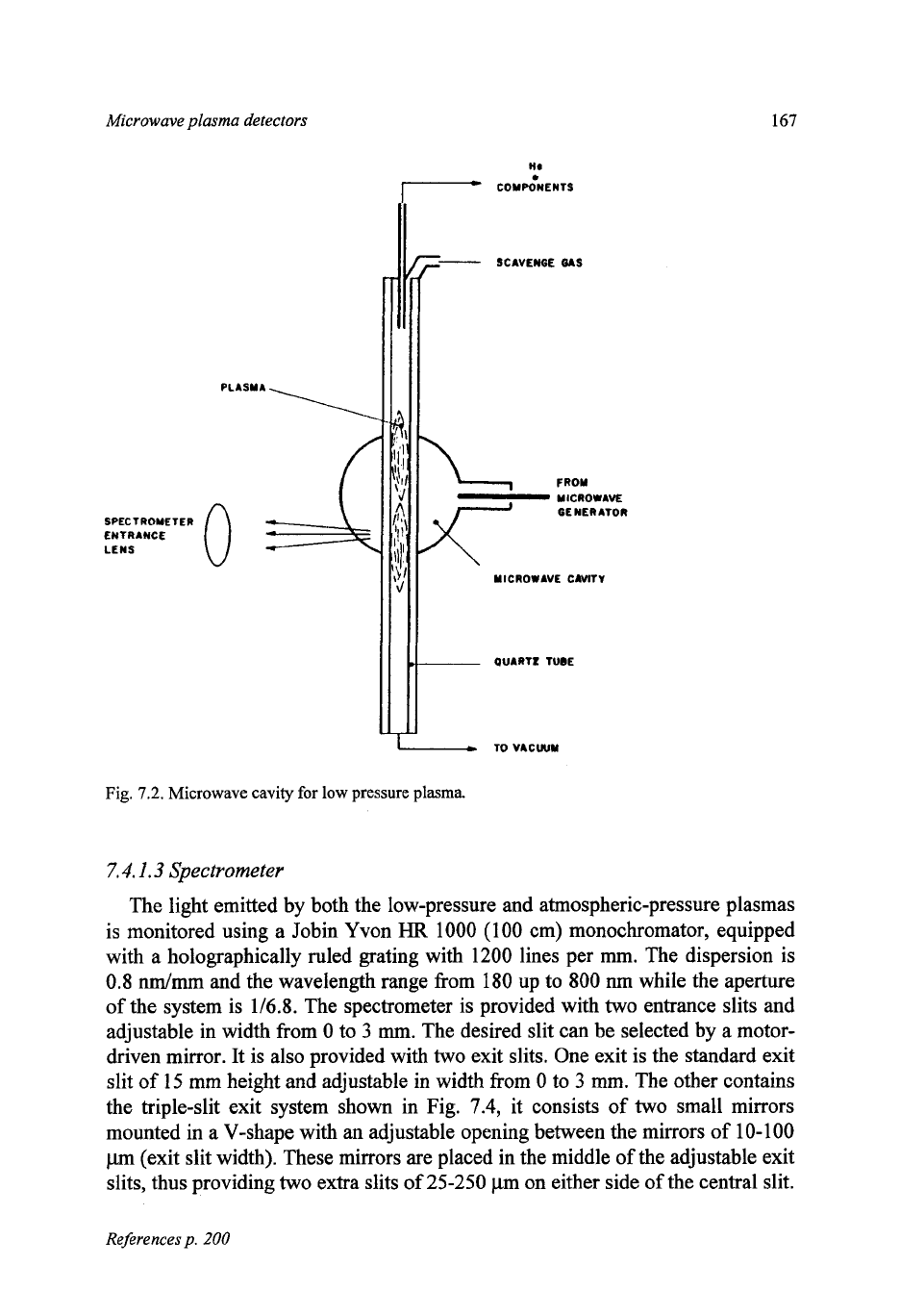
The low-pressure plasma is created
in
a quartz tube placed (Fig. 7.2) in a 114
wave
214L
Evenson type microwave cavity. It is provided with two tuning de-
vices in order to adjust the resonance frequency of the cavity to the frequency of
the microwave power supply, which
is
fixed. The quartz tube together with the
Microwave plasma detectors
165
COMPUTER
20
ml
/min
SC~VE
LOW
PRESSURE VACUUM
PUMP
PLASMA
EXPONENTIAL DILUTION FLASK
Fig.
7.1.
Schematic set-up
of
detection system.
cavity is mounted in a frame in order to adjust the plasma in the focus of the
spectrometer. The quartz tube is connected to a vacuum pump via a back-
pressure regulator and pressure monitor. The emitted radiation is monitored
sideways via the quartz wall of the tube.
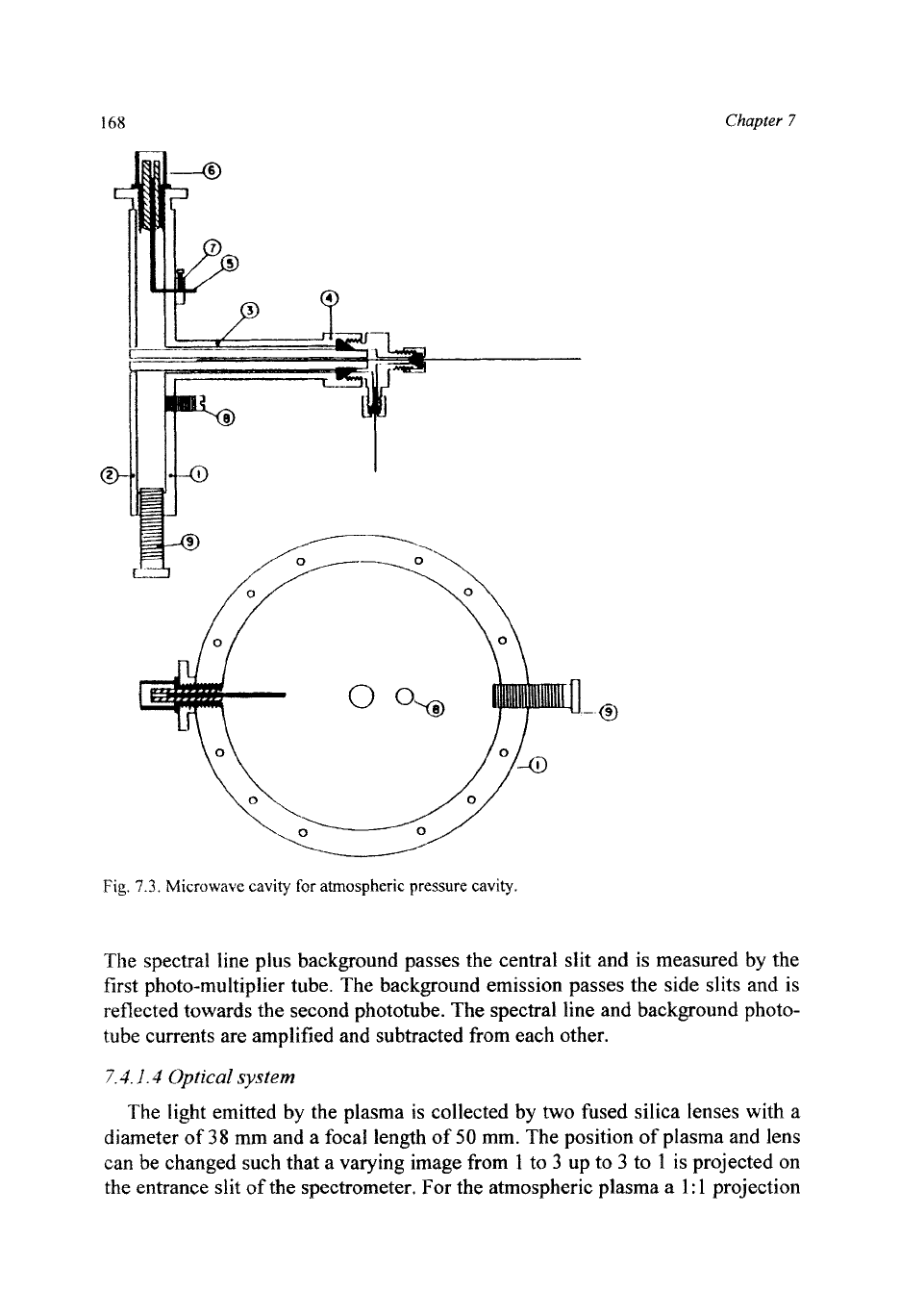
The atmospheric-pressure plasma is generated in a quartz tube in a cylindrical
cavity (Fig. 7.3), as suggested by Beenakker, in which case the internal diameter
determines the resonance frequency. The resonance frequency is independent
of
the height of the cylinder but the electric field strength increases inversely with
the height of the cylinder. Cavities with a height of
5
mm showed the best per-
formance. For a frequency of
2450
MHz
with the cavity filled with air, the inter-
nal diameter is calculated to be 93.7 mm. The quartz tube is placed axially in the
centre of the cavity where the electric field strength is maximal. The introduction
of a dielectric into the cavity shifts the resonance frequency to lower values.
Therefore
two
tuning devices were installed and the diameter was made some-
what smaller. Cavities with 92 and 93 mm could both be tuned to a very low
level of reflected power. In later versions the coupling loop fixing block (7) was
made movable and both tuning devices removed. After one final adjustment of
the coupling loop further tuning appeared to be unnecessary and the reflected
power always remained below
0.5
%. For high-temperature work a ceramic plate
was constructed between the cavity
(2)
and the inlet (3). This part can be heated
References
p.
200

I66
Chapter
7
TABLE
7.
I
DETAILS
OF
APPARATUS USED
Lenses
Monochromator
Grating
Atmospheric plasma Cavity
Helium flow rate
Quartz
tube i.d.
Microwave power
Helium flow rate
Quartz
tube id.
Microwave power
Plasma pressure
Material
Diameter
Focal length
Jobin Yvon
Slit heights
Slit widths
Holographically ruled
Lines
Dispersion
Wavelength range
Photomultipliers
Time constant
Except for
Hg/OP
As
given
in
tables
Low-pressure plasma Cavity
Amplifiers Measuring resistance
Scavenger Oxygen
Emission lines
Beenakker type, modified depth,
5
mm
30
mVmin
1.0
mm
50
W
Evenson
214
L
1/4
wavelength
30
mVmin
1.0
mm
120
w
40
mbar
Fused silica
38
mm
50
mm
HR
1000
15
nun
50
pm
120
X
140mm
1200/mm
0.8
nm/mm
180-800
nm
Hamamatsu
R446S
or
R955
1
MS2
0.1
s
0.2
and
0.02%
v/v low and atm. plasma
up
to 450°C. Air cooling is supplied via the open end
(9).
The cavity was
mounted on a movable support in order to adjust the plasma, which was moni-
tored via the open end
of
the tube,
in
the focus of the spectrometer.
7.4.1.2
Microwave power
supply
Both microwave generators are
of
the same type for both cavity types and in-
clude a high-voltage power supply
(1600-2000
V)
and a
200
W
magnetron oscil-
lator, Mullard type
7090
with a resonance frequency
of
2450
MHz.
The power
is
adjustable between 20 and
200
W.
The microwave output
of
the magnetron is
coupled to the cavity via a reflection unit, a directional coupler Narda
3003/30
and a high power coaxial cable. When the resonance frequency of the cavity
is
not exactly the same as the frequency of the generator, part of the power
is
re-
flected backwards into the generator and heats the magnetron. Therefore a re-
flected-current monitoring and overload protecting circuit
is
incorporated in or-
der to prevent damage to the magnetron if a serious mismatch occurs.
Microwave plasma detectors
167
n*
COMPONENTS
SPECTROMETER
ENTRANCE
LENS
Fig.
7.2.
Microwave cavity for
low
pressure plasma.
7.4.1.3
Spectrometer
The light emitted by both the low-pressure and atmospheric-pressure plasmas
is monitored using a Jobin Yvon
HR
1000
(100 cm) monochromator, equipped
with a holographically ruled grating with
1200
lines per mm. The dispersion is
0.8
dmm and the wavelength range from
180
up
to 800
nm
while the aperture
of the system is
1/63.
The spectrometer is provided with two entrance slits and
adjustable in width from
0
to
3
mm.
The desired slit can be selected by a motor-
driven mirror. It is also provided with two exit slits. One exit is the standard exit
slit
of
15
mm
height and adjustable in width from
0
to
3
mm. The other contains
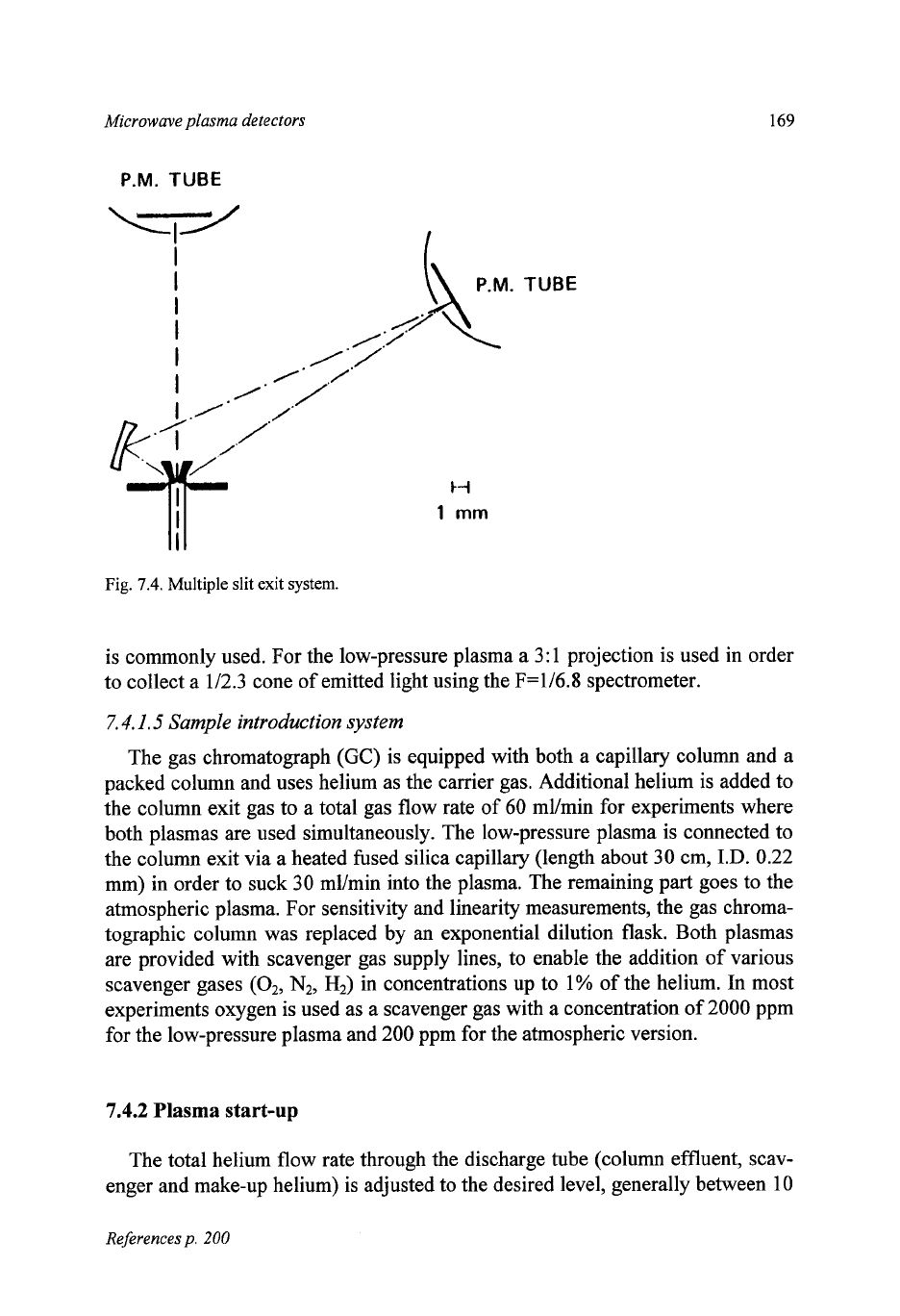
the triple-slit exit system shown in Fig.
7.4,
it consists of
two
small mirrors
mounted in a V-shape with an adjustable opening between the mirrors of 10-100
pm
(exit slit width). These mirrors are placed in the middle
of
the adjustable exit
slits, thus providing
two
extra slits
of
25-250
pm on either side of the central slit.
References p.
200
Chapter
7
I68
Fig.
7.3.
Microwave cavity
for
atmospheric
pressure
cavity.
8
The spectral line plus background passes the central slit and is measured by the
first photo-multiplier tube. The background emission passes the side slits and is
reflected towards the second phototube. The spectral line and background photo-
tube currents are amplified and subtracted from each other.
7.3.1.3
Optical
system
The light emitted by the plasma is collected by
two
fused silica lenses with
a
diameter of
38
mm and a focal length of
50
mm. The position of plasma and lens
can be changed such that a varying image from
I
to
3
up to
3
to
1
is projected
on
the entrance slit
of
the spectrometer. For the atmospheric plasma a
1
:
1
projection
Microwave plasma detectors
169
P.M.
TUBE
I
0’
/.
/
t-i
1
mm
Fig.
7.4.
Multiple slit exit system.
is commonly used. For the low-pressure plasma a
3:
1 projection is used in order
to collect a
U2.3
cone of emitted light using the F=1/6.8 spectrometer.
7.4.
I.
5
Sample introduction system
The gas chromatograph
(GC)
is equipped with both a capillary column and a
packed column and uses helium as the carrier gas. Additional helium is added to
the column exit gas to a total gas flow rate of 60 mumin for experiments where
both plasmas are used simultaneously. The low-pressure plasma is connected to
the column exit via a heated fused silica capillary (length about
30
cm,
I.D.
0.22
mm) in order to suck
30
ml/min into the plasma. The remaining part goes to the
atmospheric plasma. For sensitivity and linearity measurements, the gas chroma-
tographic column was replaced by an exponential dilution flask. Both plasmas
are provided with scavenger gas supply lines, to enable the addition of various
scavenger gases
(02,
NZ,
H2)
in concentrations up to 1% of the helium. In most
experiments oxygen is used as a scavenger gas with a concentration
of
2000
ppm
for the low-pressure plasma and
200
ppm for the atmospheric version.
7.4.2
Plasma start-up
The total helium flow rate through the discharge tube (column effluent, scav-
enger and make-up helium) is adjusted to the desired level, generally between 10
References
p.
200

170
Chapter
7
and
50
ml/min and the microwave power set to about
50
W
for the atmospheric-
pressure plasma and 120
W
for the low-pressure plasma. The APP is then initi-
ated by momentarily inserting the tip of
a
small-diameter wire, held in
a
piece of
insulator, into the open end of the discharge tube. Plasma is initiated and stable
within a second. For the LPP a spark produced by a Tesla coil is used. After the
first ignition of the plasma the reflected power
is
turned to
a
minimum by mov-
ing the coupling loop a little in or
out
the cavity to find a position with minimum
reflected power as measured by the generator. Under normal operating condi-
tions the reflected power
is
less than
0.2
W
at
a
forward power of
50
W.
After
a
few seconds the plasma itself has stabilized but it takes about
15
minutes for the
cavity
to
obtain a constant temperature and a slight retuning is sometimes re-
quired. For safety reasons the microwave radiation around the cavities was
monitored with an electromagnetic leakage monitor model 8201 of the “Narda
Microwave Corporation”. The stray radiation appeared to be less than
0.05
mW/cm2 at a distance of
10
cm from the cavity, which is well within the safety
limits. Only in front of the open end is the radiation level higher and the area
between lens and cavity must be covered.
7.4.3
Operating limits
of
the detector
Gas chromatographic detectors can be classified into
two
categories. For the
first
group of detectors, the signal at any moment depends on the concentration
of the sample in the carrier gas. The sensitivity of these detectors, including the
well-known thermal conductivity detector, should be expressed
as
signal per unit
of
concentration.
In the second group of detectors, the signal
is
dependent on the mass flow rate
of
the sample. The response of the flame ionization detector, for example, is
therefore expressed in signal per mass flow rate of sample. For both plasma
types, the response is almost constant over
a
column flow-rate from
5
to
50
ml/min of He. This clearly indicates that the atomic emission detector
is
a
concentration-type detector. The minimal flow rate for the APP is limited by
upstream diffusion of air from the open end of the plasma tube.
The linear dynamic range (LDR)
of
the detector
is
the span over which the
detector exhibits a constant sensitivity to various and increasing sample concen-
trations. The lower end of this range
is
called minimum detectable level (MDL).
It
is
generally accepted that the smallest detectable amount of element is that
quantity which produces a signal equal to twice the bandwidth
of
the background
variations. Some parameters influencing the MDL and the LDR are discussed.
The upper detection limit (UDL)
is
attained when the signal is no longer line-
arly proportional
to
the amount of sample introduced into the detector. This up-
Microwave plasma detectors
171
per limit is not
a
fixed point; the signal obtained begins to deviate from the ex-
trapolated linear relationship. When this deviation is more than a certain per-
centage (generally
5%),
the upper limit of detection is reached. The ratio be-
tween upper limit and lower detection limit is generally expressed in decades
and should be as large as possible, e.g. larger than
4
decades. Two important pa-
rameters influencing the UDL will be mentioned, viz. the concentration
of
atoms
not measured in the plasma, and the type and amount of scavenger gas.
Concentrations above the UDL can be introduced into the plasma, but the sig-
nal is no longer proportional to the concentration, carbon
is
deposited onto the
wall of the quartz tube and finally the plasma extinguishes. Moreover, these high
concentrations also decrease the signal produced by other elements, which them-
selves are within their LDR.
7.4.3.1
Emission line intensity
Helium is used as carrier gas because it has the highest excitation energy of
all elements and therefore all other elements can, in principle, be detected. Each
element that can be detected by atomic emission, has more than one emission
line between
180
and
800
nm from which of course the most intense lines pro-
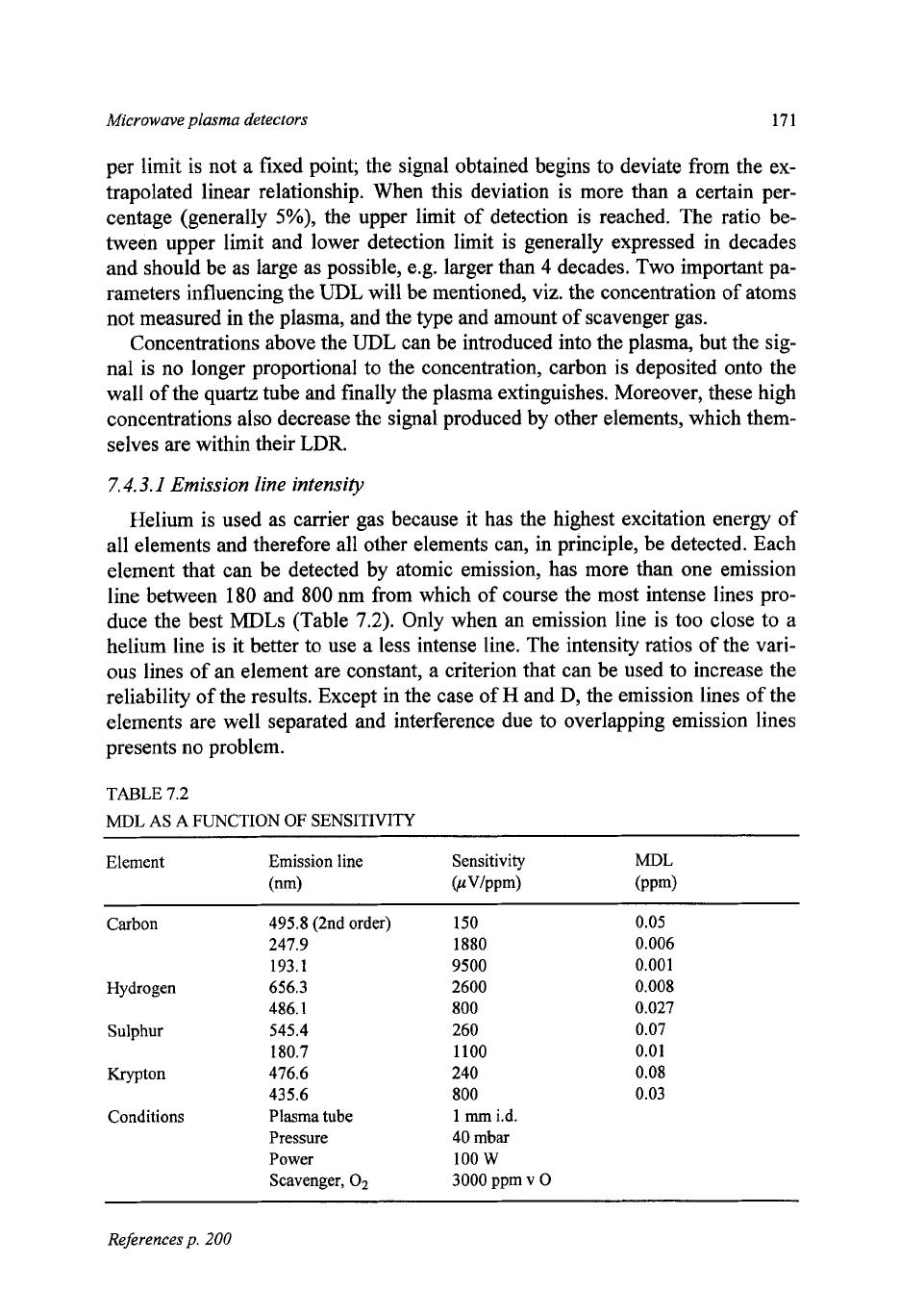
duce the best MDLs (Table
7.2).
Only when an emission line
is
too close to a
helium line is it better to use
a
less intense line. The intensity ratios of the vari-
ous lines of an element are constant, a criterion that can be used to increase the
reliability of the results. Except in the case of
H
and D, the emission lines of the
elements are well separated and interference due to overlapping emission lines
presents no problem.
TABLE
7.2
MDL
AS
A
FUNCTION OF SENSITIVITY
Element Emission line Sensitivity
h4DL
(nm)
OIVlppm) (PP@
Carbon
495.8 (2nd
order)
150 0.05
247.9 1880 0.006
193.1 9500
0.001
Hydrogen
656.3
2600 0.008
486.1
800 0.027
Sulphur
545.4
260 0.07
180.7
1100
0.01
Krypton
476.6
240
0.08
435.6
800 0.03
Conditions Plasma tube
1
mm
i.d.
Pressure
40
mbar
Power
100
w
Scavenger,
O2
3000
ppm v
0
References p.
200
