Yeang K., Woo L. Dictionary of Ecodesign: An Illustrated Reference
Подождите немного. Документ загружается.

Urban and suburban developments and their
accompanying paved roads and surfaces have
increased the probabilities of flooding.
On-peak energy Energy supplied during periods
of relatively high system demand as specified by
the supplier. See also:
Off peak
Once-through system design strategy One of
four design strategies to manage materials and
energy in the built form and its servicing sys-
tems. Resources are consumed with the belief
that they are unlimited. Once they have fulfilled
their usefulness, they are discarded as waste. Uses
the environment as its ultimate sink. See also:
Closed-circuit system design strategy; Combined
open-circuit system design strategy; Open-circuit
system design strategy
Open access The ability to send or wheel
electric power to a customer over a transmission
and distribution system that is not owned by the
power generator (seller).
Open access system Also known as the “tragedy
of the commons”. Commonly held resource for
which there are no management rules. Experience
has shown that common resources are often
exploited or degraded by some of the owners.
Open-circuit system design strategy One of
four design strategies to manage materials and
energy in buildings and their servicing systems.
Like the once-through system, it uses the envir-
onment as a sink to receive waste products, but
in this system the emissions do not exceed the
ability of the ecosystem to absorb them. The
wastes are pretreated before they are discharged.
See also:
Closed-circuit system design strategy;
Combined open-circuit system design strategy;
Once-through system design strategy
Open-loop active system Solar water heating
system. Pumps circulate water through the solar
collectors. These systems are most effective in
geographical areas that do not freeze for long
periods and do not have hard or acidic water.
Open-loop geothermal heat pump system Also
known as a direct system. Circulates water drawn
from a ground or surface water source. Once
the heat has been transferred into or out of the
water, the water is returned to a well or surface
discharge (instead of being recirculated through
the system). This option is practical where there
is an adequate supply of relatively clean water,
and all local codes and regulations regarding
groundwater discharge are met.
Open system Area in which there is a supply
of chemical elements used to support plant
and animal communities in one ecosystem
and a simultaneous loss of biomass and che-
mical elements from the area outside of that
ecosystem.
Operational costs Direct monetary costs of
operating a business or facility.
Organic Contains carbon, and is composed of
living or previously living matter.
Organic agriculture Concept and practice of
agricultural production without the use of syn-
thetic inputs and without allowing the use of
transgenic organisms.
Organic compound Compound containing
carbon chains or rings and hydrogen with or
without oxygen, nitrogen and other elements;
foundation of modern polymer chemistry. See also:
Inorganic compound; Volatile organic compound
Organic cyanides Toxic chemicals. Any chemi-
cal compound containing the combining group
CN. Cyanide is highly toxic and fast-acting: it
inhibits cells’ oxidative processes. Cyanides
occur naturally in certain seeds, such as apple
168 On-peak energy
seeds and wild cherry pits. Cyanides, including
hydrogen cyanide (HCN or hydrocyanic acid),
are used industrially in the production of acrylic
fibers, synthetic rubbers, and plastics as well as
in electroplating, case-hardening of iron and
steel, fumigation, and concentration of ores. See
also:
Toxic chemicals
Organic waste Materials that decompose
naturally.
Organically grown Agricultural crops grown
without the use of synthetic fertilizers or pesticides.
Organism Anything that is living, from bacteria
to plants to animals.
Orientation Orientation of a surface in degrees
away from solar south, towards either east or
west. Solar or true south is distinct from magnetic
south.
Oriented-strand board (OSB) An engineered,
reconstituted mat-formed building material panel
made of strands, flakes, or wafers sliced from
small-diameter, round wood logs and bonded
with an exterior-type binder under heat and pres-
sure. Exterior or surface layers are composed of
strands aligned in the long panel direction;
inner layers consist of cross-aligned or randomly
aligned strands.
Osmotroph Organism that obtains nutrients
through its cell membrane. Examples are fungi
and bacteria, which can’t use particulate matter
as nutrients. See also:
Phagotroph
OTEC See: Ocean thermal energy conversion
Outage A discontinuance of electric power
supply.
Outfall Site where effluent is discharged into
receiving waters.
Outgassing The process by which materials
expel or release gases.
Overhang Building element that shades win-
dows, walls, and doors from direct solar radiation
and protects these elements from precipitation.
Overload To exceed the design capacity of a
device.
Ovonic This word, invented by Standford
Ovshinsky, is composed of OVshinsky and
electrONIC. Refers to amorphous glassy semi-
conductors (germanium, tellurium, arsenic) that
change reversibly from an electrically noncon-
ducting state to a conducting state under applica-
tion of an electric field of the order of 10.5 V/cm.
Oxidant Also known as an oxidizer agent.
Chemical, such as oxygen, that consumes electrons
in an electrochemical reaction.
Oxidation 1. Mixture of a substance with
oxygen; rust or burning may result.
2. Process in which electrons are removed
from atoms or ions.
Oxidation pond Human-made body of water in
which waste is consumed by bacteria. Used most
frequently with other waste-treatment processes.
Could be a sewage lagoon.
Oxidize 1. Chemically transform a substance
by combining it with oxygen.
2. Remove electrons from atoms or ions.
Oxidizer agent See:
Oxidant
Oxygen cycle Circulation of oxygen through
environmental compartments. It is closely linked
to the carbon cycle.
Oxygenated fuel (oxyfuel) Special type of
gasoline that burns more completely than
Oxygenated fuel (oxyfuel) 169
regular gasoline in cold-start conditions. It pro-
duces reduced carbon dioxide compared with
regular gas.
Oxygenate To treat, combine, or infuse with
oxygen.
Oxygenates Substances which, when added
to gasoline, increase the amount of oxygen in
the gasoline blend. Ethanol, methyl tertiary
butyl ether (MTBE), ethyl tertiary butyl ether
(ETBE), and methanol are common oxygenates.
Additives to gasoline reduce its carbon dioxide
emissions.
Ozone (O
3
) A major air pollutant, ozone gas is
a triatomic form of oxygen. It is not usually
emitted directly into the air, but at ground level
it is created by a chemical reaction between
oxides of nitrogen (NO
x
) and volatile organic
compounds (VOC) in the presence of sunlight.
Ozone has the same chemical structure whe-
ther it occurs miles above the Earth or at ground
level. It is produced in two layers: the tropo-
sphere or ground-layer ozone, known as “bad”
ozone; and the stratosphere or upper ozone,
known as “good” ozone.
Ozone is a primary constituent of smog.
Motor vehicle exhaust and industrial emissions,
gasoline vapors, and chemical solvents, as well
as natural sources, emit NO
x
and VOC that help
form ozone. Sunlight and hot weather cause
ground-level ozone to form in harmful con-
centrations in the air. As a result, it is known as
a summertime air pollutant. Ozone occurs in
both urban and rural areas. It is considered a
significant health risk, particularly for children
with asthma. It also damages crops, trees, and
other vegetation.
Ozone is produced naturally in the stratosphere.
“Good” ozone occurs naturally in the strato-
sphere approximately 10–30 miles (16–48 km)
above the Earth’s surface, and forms a layer that
protects life on Earth from the Sun’s harmful
ultraviolet (UV) rays. This natural shield has gradu-
ally been damaged or depleted by human-made
chemicals including chlorofluorocarbons (CFCs),
hydrochlorofluorocarbons (HCFCs), halons, methyl
bromide, carbon tetrachloride, and methyl chloro-
form, all known as ozone-depleting substances.
Even though there has been a reduction in
the use of many ozone-depleting substances,
their use in the past can still affect the protective
ozone layer. Research indicates that depletion
of the “good” ozone layer is being reduced
worldwide. Thinning of the protective ozone
layer can be observed using satellite measure-
ments, particularly over the polar regions.
Ozone depletion can cause increased amounts
of UV radiation to reach the Earth, which can
lead to more cases of skin cancer, cataracts, and
impaired immune systems. Measures are being
taken to reduce ozone emissions in the USA
through the Clean Air Act, and also in other
countries. See also:
Air pollutants; ozone-depleting
substance
Ozone-depleting substance (ODS) Family of
human-made compounds that have been shown
to deplete stratospheric ozone. ODS include
chlorofluorocarbons (CFCs), bromofluorocarbons
(halons), methyl chloroform, carbon tetrachloride,
methyl bromide, and hydrochlorofluorocarbons
(HCFCs). Once released into the air, these ozone-
depleting substances degrade very slowly. They
can remain intact for years as they move through
the troposphere until they reach the stratosphere.
There they are broken down by the intensity of
the Sun’s ultraviolet rays and release chlorine
and bromine molecules, which destroy the “good”
ozone. Scientists estimate that one chlorine atom
can destroy 100,000 “good” ozone molecules.
See also:
Ozone
Ozone hole Thin place in the ozone layer in
the stratosphere. Thinning of the stratospheric
ozone has been linked to the destruction of stra-
tospheric ozone by chlorofluorocarbons (CFCs)
170 Oxygenate
and related chemicals. Reductions in ozone levels
will lead to higher levels of ultraviolet B (UVB)
reaching the Earth’s surface. The Sun’s output of
UVB does not change; rather, less ozone means
less protection, and hence more UVB reaches the
Earth. Studies have shown that in the Antarctic,
the amount of UVB measured at the surface can
double during the annual ozone hole. Another
study confirmed the relationship between reduced
ozone and increased UVB levels in Canada
during the past several years.
Ozone layer The layer of ozone that begins
approximately 15 km (9.3 miles) above Earth
and thins to an almost negligible amount at
about 50 km (31 miles), and shields the Earth
from harmful ultraviolet radiation from the Sun.
The highest natural concentration of ozone
(approximately 10 parts per million by volume)
occurs in the stratosphere at approximately 25
km (15.5miles) above Earth. The stratospheric
ozone concentration changes throughout the
year as stratospheric circulation changes with
the seasons. Natural events such as volcanoes
and solar flares can produce changes in ozone
concentration, but human-made changes are of
the greatest concern. The ozone layer protects
the Earth against most ultraviolet (UV) radiation
coming from the Sun. UVB is a type of ultra-
violet light from the Sun (and sunlamps) that
has several harmful effects, particularly in
damaging DNA. It is a cause of melanoma and
other types of skin cancer. Studies have found
adverse effects on plants, materials, and marine
organisms from excessive exposure to UV.
Solar UVB radiation has been found to cause
damage to early developmental stages of fish,
shrimp, crab, amphibians, and other animals.
The most severe effects are decreased repro-
ductive capacity and impaired larval develop-
ment. Even at current levels, solar UVB
radiation is a limiting factor, and small increases
in UVB exposure could result in significant
reduction in the size of the population of ani-
mals that eat these smaller creatures. See also:
Stratosphere, Ultraviolet radiation
Ozone precursors Chemical compounds, such
as carbon monoxide, methane, nonmethane
hydrocarbons, and nitrogen oxides, which react
with the Sun’s radiation and other chemical
compounds to form ozone. See also:
Troposphere
Ozone precursors 171
P
Packaging Wrappers for products; wrappers
may be made of a variety of materials: plastic,
cardboard, paper, metal, glass, wood, and
ceramics.
Packed tower Also known as packer tower
scrubber. Air pollution control device. Con-
taminated air is circulated through a tower
containing materials that have a large surface
area. The contaminants are absorbed into a liquid
flowing over the tower materials. The liquid falls
downward and the air is forced upward through
the tower. The vapors or particulates contained
in the air go up through the surface area. The
fumes come into contact with the surface and
take up the material releasing the air and
cleaning it. See also:
Absorption process; Scrubbers;
Ventury scrubber
Packed tower aeration Process to remove
organic contaminants from groundwater. Ground-
water flows downward in a tower filled with
material over a large surface. Air is introduced at
the bottom of the tower and is forced upward past
the falling groundwater. Organic contaminants
are transferred from the water to the air.
Packer tower scrubber See:
Packed tower
PAFC See: Phosphoric acid fuel cell
Panemone A type of vertical-axis wind turbine.
It has a rotating axis positioned at 90° to the
direction of the wind, while the wind-catching
blades move parallel to the wind. By contrast,
the shaft of a horizontal-axis wind turbine points
into the wind while its blades move at right-angles
to the wind’s thrust. A panemone primarily uses
drag, whereas the blades of a horizontal-axis
wind turbine use lift. The panemone windmill
dates from ancient Persia, where it was used as
a power source to grind wheat. It is drag-type
wind machine that can react to wind from any
direction.
Paper Product made from tree pulp in a process
that produces acid rain and dioxin.
Parabolic aluminized reflector lamp A type of
lamp with a lens of heavy, durable glass that
focuses the light. They have longer lifetimes
with less lumen depreciation than standard
incandescent lamps.
Parabolic dish solar collector A solar energy
conversion device that has a bowl-shaped dish
covered with a highly reflective surface that
tracks the Sun and concentrates sunlight on a
fixed absorber, thereby achieving high tempera-
tures: for process heating, or to operate a heat
(Stirling) engine to produce power or electricity.
See also:
Stirling engine
Parabolic trough A type of solar thermal col-
lector. A solar energy conversion device that
uses a trough covered with a highly reflective
surface of either coated silver or polished alu-
minum to focus sunlight onto a linear absorber,
usually a Dewar tube, containing a working
fluid that absorbs the concentrated sunlight. The
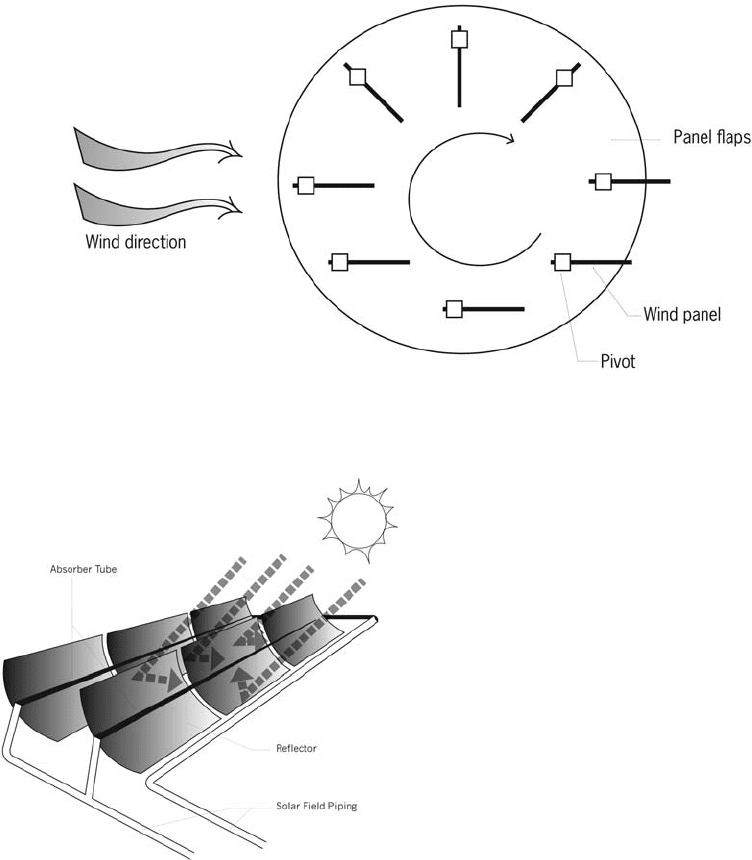
heat-transfer fluid, usually oil, is used to heat
steam in a standard turbine generator. Estimates
of its economic and thermal efficiency range
from 60 to 80%. See also:
Solar thermal electric
systems
At present, all parabolic troughs are hybrids.
They include a fossil fuel system to supplement
the solar energy at night or when it is cloudy.
The fossil fuel is usually natural gas.
Parasitism Form of symbiosis in which one
species benefits and the other is harmed. See
also:
Amensalism; Commensalism; Mutualism;
Symbiosis
Partial zero-emission vehicle (PZEV)
Designation by the California Air Resources Board
for a vehicle that has zero evaporative emissions
from its fuel system, has a 15 year (or at least
150,000 mile) warranty, and meets super-ultra-
low-emission vehicle (SULEV) tailpipe emissions
standards. A PZEV falls into the SULEV category.
See also:
Emissions standards, designations
Particulate matter Also known as particle
pollution or PM. Common air pollutant, toxic
chemical, and health threat. A complex mix-
ture of extremely small particles and liquid
droplets. Particle pollution is made up of a
number of components, including acids (such
as nitrates and sulfates), organic chemicals,
metals, and soil or dust particles. Particulate
pollution can cause health problems, especially
Figure 49 A parabolic trough collector
Source: US Department of Energy
Figure 48 A panemone—wind-catching panels turn edge-on to the wind when moving against the wind’s
thrust and side-on when moving downwind
Particulate matter 173
to the respiratory system. See also: Air pollutants;
Toxic chemicals
Particulates Small particles that are suspended
in air, such as soot, ash, dust, or other air emissions.
See also:
Air emissions
Partitioned matrix (LP) Developed by ecode-
signers to depict the interactions between a
designed system and the environment. Using 1
for the designed system, 2 for the environment,
and L for the interdependencies within a given
framework, the matrix is:
L
11
= processes that occur within the system
(internal interdependencies); L
22
= activities in
the environment (external interdependencies);
L
12
and L
21
= system/environment and environment
system exchanges.
Passive cooling systems Various simple cool-
ing techniques designed to lower indoor tem-
perature through the use of natural energy
sources. Design considerations include layout
of building, orientation, number, size, location,
and details of windows, shading devices, ther-
mal resistance, and heat capacity of the build-
ing envelope. The designs involve minimizing
heat gain by the building, minimizing solar
heat build-up of the building envelope and
solar penetration through windows, and provid-
ing natural ventilation. Minimizing heat gain
has not traditionally been regarded as a cooling
technology. Passive cooling systems typically
involve deep sky cooling or other cooling with-
out using motors to move a fluid to transport the
heat (or cool) liquids. See also:
Passive solar
cooling
Passive daylight device Nonmechanical device
used to project daylight into the interior of a
structure. See also:
Light pipe; Light shelf; Tubular
skylight
Passive diffuser Air supply outlet, without a
fan, that relies on pressurized plenum or duct
air to deliver air into the conditioned space of a
building.
Passive mode design Also known as bioclimatic
design. An ecodesign strategy that specifies low-
energy consumption systems by taking advan-
tage of the ambient climate of the locality. Pas-
sive mode ecodesign emphasizes three main
design considerations: i) low-energy design; ii)
the climate of the locality and the site ’s natural
features; iii) the appropriate shape of a building
and its ratio of volume to surface. In design
terms, passive mode design strategies include
appropriate built form configuration and orien-
tation, internal spatial disposition, façade design,
such as solid to glass ratio, insulation, and color,
use of building mass, use of vegetation, natural
ventilation, and even color. Passive mode
design as low-energy design seeks to maximize
natural and ambient energy sources and mini-
mize the use of nonrenewable energy resources.
This can be achieved through the use of radia-
tion, conduction, and convection instead of
electromechanical heating and cooling systems
that require external sources of energy.
The climate and locality and the site’s natural
features influence the kind of design for the
built form, but do not determine the design. The
design is a response to the prevailing climate
and seasons over the year. Designs for tempe-
rate and cold climates are necessarily different,
for example, just as the specific site’s topo-
graphy and neighbors influence the design. The
appropriate shaping of the building and its
orientation are determined by the locality’s Sun
path, use of natural ventilation, use of vegeta-
tion, appropriate façade design, and Sun shad-
ing and other similar considerations. Passive
mode design relates to the climatic conditions
174 Particulates
of the locality and results in built forms that are
more adapted to a specific region.
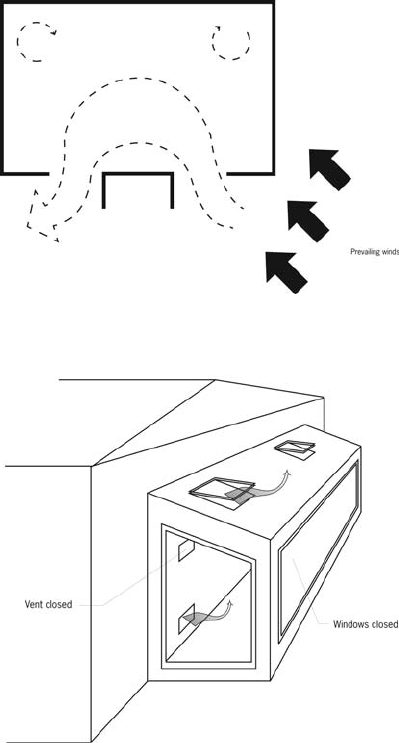
Passive solar cooling Use of natural ventila-
tion is a primary strategy for cooling buildings
without mechanical assistance in hot humid
climates. Methods to increase the effectiveness
of natural ventilation include the following:
operable windows—should be placed on the
south exposure
wing walls—vertical solid panels placed
alongside windows perpendicular to the wall
on the windward side of the house
thermal chimneys
sunrooms.
Sunrooms can be designed to perform this func-
tion by venting at the top any excessive heat
generated in a south-facing sunroom during
the summer. With the connecting lower vents to
the living space open along with windows on the
north side, air is drawn through the living space
to be exhausted through the sunroom upper
vents. (The upper vents from the sunroom to the
living space and any side operable windows
must be closed and the thermal mass wall in the
sunroom must be shaded.) Thermal mass indirect
gain walls can be made to function similarly,
except that the mass wall should be insulated
on the inside when performing this function.
Thermal chimneys can be constructed in a narrow
configuration (like a chimney) with an easily
heated black metal absorber on the inside behind
a glazed front that can reach high temperatures
and be insulated from the house. The chimney
must terminate above the roof level. A rotating
metal scoop at the top, which opens opposite
the wind, will allow heated air to exhaust with-
out being overcome by the prevailing wind.
Thermal chimney effects can be integrated into
houses with open stairwells and atria.
Other ventilation strategies include making
the outlet openings slightly larger than the inlet
openings; and placing inlets at low-to-medium
heights to provide airflow at occupant levels in
the room. Inlets close to a wall result in air
“washing” along the wall; it is important to have
centrally located inlets for air movement in the
center areas of the room. Window insect
screens decrease the velocity of slow breezes
more than stronger breezes (60% decrease at
1.5 mph (2.4 km/ph), 28% decrease at 6 mph
(9.6 km/ph)). Screening a porch will not reduce
air speeds as much as screening the windows.
Night ventilation of a home should be done at a
Figure 50b Passive solar cooling
Figure 50a Passive solar cooling
Passive solar cooling 175
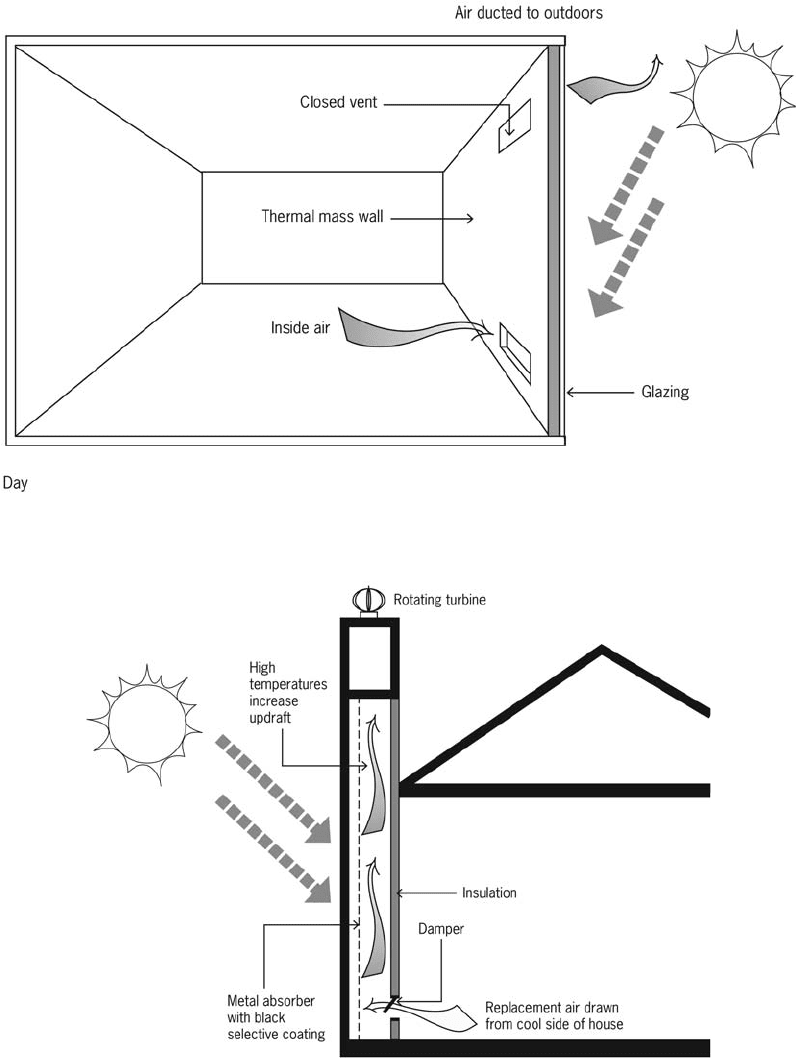
Figure 50d Passive solar cooling
Figure 50c Passive solar cooling
176 Passive solar cooling
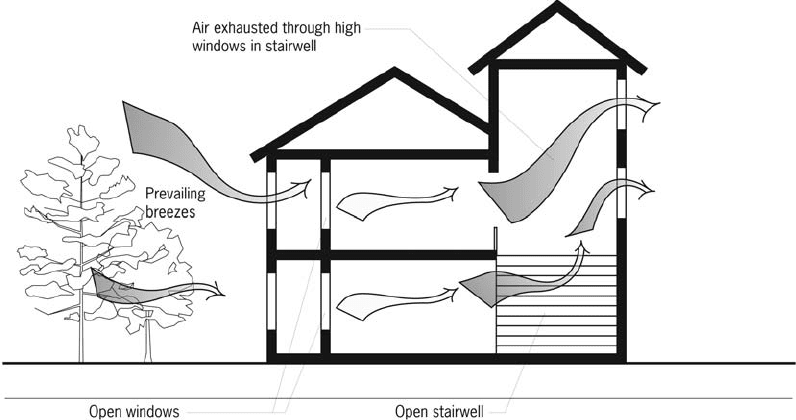
ventilation rate of 30 air changes per hour or
greater. Mechanical ventilation will be required
to achieve this. High-mass houses can be cooled
with night ventilation provided fabric furnish-
ings are minimized in the house. Keep a high-
mass house closed during the day and opened at
night. See also:
Passive cooling systems; Thermal
chimney; Wing wall
Passive solar design Design that uses the Sun’s
energy for heating and cooling of living spaces.
In this approach, the building itself or some
element of it takes advantage of natural energy
characteristics in materials and air created by
exposure to the Sun to heat and cool the building
without use of mechanical equipment. Passive
systems are simple, have few moving parts, and
require no mechanical systems and minimal
maintenance.
Principal design elements include proper
building orientation, window sizing, placement
and design of window overhangs to reduce
summer heat gain and ensure winter heat gain,
and proper sizing of thermal energy storage mass
such as a Trombe wall or masonry tiles. The
heat is distributed primarily by natural convec-
tion and radiation, although fans can also be used
to circulate room air or ensure proper ventila-
tion. Operable windows, thermal mass, thermal
chimneys, and wing walls are common elements
found in passive design. Wing walls are vertical
exterior wall partitions placed perpendicular to
adjoining windows to enhance ventilation through
windows. See also:
Thermal chimney; Thermal
mass; Trombe wall; Wing wall
Passive solar energy system General term
used to designate solar heating or cooling that
uses natural energy flows to transfer heat.
Passive solar heater A solar water or space-
heating system in which solar energy is collected,
and/or moved by natural convection without
using pumps or fans. Passive systems are usually
integral collector/storage or batch collectors, or
thermosiphon systems. These systems do not use
controls, pumps, sensors, or other mechanical
parts, so little or no maintenance is required
over the lifetime of the system. See also:
Batch
heater; Thermosiphon
Figure 50e Passive solar cooling
Passive solar heater 177
Openmirrors.com
