Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

a major influence in determining their conformations. Lon-
don forces also provide much of the binding energy in the
sterically complementary interactions between proteins
and the molecules that they specifically bind.
B. Hydrogen Bonding Forces
Hydrogen bonds , as we discussed in Section
2-1Aa, are predominantly electrostatic interactions (but
with ⬃10% covalent character) between a weakly acidic
donor group (D¬H) and an acceptor (A) that bears a lone
pair of electrons. In biological systems, D and A can both
be the highly electronegative N and O atoms and occasion-
(D¬H
p
A)
ally S atoms. In addition, a relatively acidic C¬H group
(e.g., a C
␣
¬H group) can act as a weak hydrogen bond
donor, and the polarizable electron system of an aro-
matic ring (e.g., that of Trp) can act as a weak acceptor.
Hydrogen bonds have association energies that are nor-
mally in the range ⫺12 to ⫺40 kJ ⴢ mol
⫺1
(but only around
⫺8 to ⫺16 kJ ⴢ mol
⫺1
for and hydro-
gen bonds and ⫺2 to ⫺4 kJ ⴢ mol
⫺1
for hydrogen
bonds), values which are between those for covalent bonds
and van der Waals forces. Hydrogen bonds (H bonds) are
much more directional than are van der Waals forces but
less so than are covalent bonds. The distance is nor-
mally in the range 2.7 to 3.1 Å, although since H atoms are
unseen in all but the very highest resolution macromolecu-
lar X-ray structures, a possible interaction
(where D and A are either N or O) is assumed to be a H
bond if its distance is significantly less than the 3.7
Å sum of a D¬H bond length (⬃1.0 Å) and the van der
Waals contact distance between H and A (⬃2.7 Å). Keep in
mind, however, that there is no rigid cutoff distance beyond
which H bonds cease to exist because the energy of an H
bond, which is mainly electrostatic in character, varies in-
versely with the distance between the negative and positive
centers (Eq. [8.1]).
H bonds tend to be linear, with the D¬H bond pointing
along the acceptor’s lone pair orbital (or, in
hydrogen bonds, roughly perpendicular to the aromatic
ring and pointing at its center with the distance from the D
atom to the center of the aromatic ring normally in the
range 3.2–3.8 Å). Large deviations from this ideal geome-
try are not unusual, however. For example, in the H bonds
of both ␣ helices (Fig. 8-11) and antiparallel  pleated
sheets (Fig. 8-16a), the N¬H bonds point approximately
along the bonds rather than along an O lone pair
orbital, and in parallel  pleated sheets (Fig. 8-16b), the H
bonds depart significantly from linearity. Indeed, many of
the H bonds in proteins are members of networks in which
each donor is H bonded to two acceptors (a bifurcated hy-
drogen bond) and each acceptor is H bonded to two donors.
For example, although the H bonds in ideal ␣ helices form
between the N¬H group at residue n and the group
at residue n ⫺ 4 (n S n ⫺ 4 H bonds; Fig. 8-11), many of the
N¬H groups in real ␣ helices associate via bifurcated H
bonds with two adjacent groups to form both
n S n ⫺ 4 and n S n ⫺ 3 H bonds.
a. Hydrogen Bonds Only Weakly Stabilize Proteins
A protein’s internal H bonding groups are arranged
such that most possible H bonds are formed (Section 8-3B).
Clearly, H bonding has a major influence on the structures
of proteins. However, an unfolded protein makes most of
its H bonds with the water molecules of the aqueous sol-
vent (water, it will be recalled, is a strong H bonding donor
and acceptor).The free energy of stabilization that internal
H bonds confer on a native protein is therefore equal to the
difference in the free energy of H bonding between the
native protein and the unfolded protein. Consequently, it
might be expected that H bonds do not stabilize (and per-
haps even slightly destabilize) the structure of a native
C “ O
C “ O
C “ O
D¬H
p
D
p
A
D¬H
p
A
D
p
A
C¬H
p
D¬H
p
C¬H
p
A
Section 8-4. Protein Stability 261
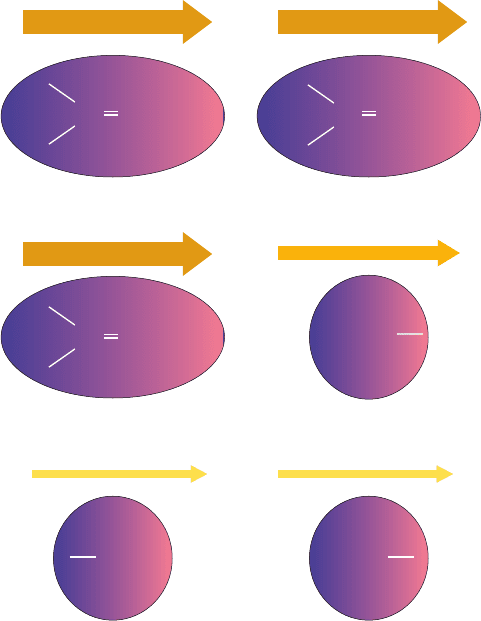
Figure 8-58 Dipole–dipole interactions. The strength of each
dipole is represented by the thickness of the accompanying
arrow. (a) Interactions between permanent dipoles.These
interactions, here represented by carbonyl groups lined up head
to tail, may be attractive, as shown, or repulsive, depending on
the relative orientations of the dipoles. (b) Dipole–induced
dipole interactions.A permanent dipole (here shown as a
carbonyl group) induces a dipole in a nearby group (here
represented by a methyl group) by electrostatically distorting its
electron distribution (shading).This always results in an attractive
interaction. (c) London dispersion forces. The instantaneous
charge imbalance (shading) resulting from the motions of the
electrons in a molecule (left) induces a dipole in a nearby group
(right); that is, the motions of the electrons in neighboring groups
are correlated.This always results in an attractive interaction.
(a)
++––
Interactions between permanent dipoles
(b) Dipole–induced dipole interactions
++––
(c) London dispersion forces
++––
C O
CH
3
H
3
C
H
3
C
δ + δ – δ + δ –
δ + δ –
δ + δ – δ + δ –
C O
C O
δ + δ –
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 261
protein relative to its unfolded state. However,since H bond-
ing interactions are largely electrostatic in nature, they are
likely to be stronger in the low polarity interior of a protein
than they are in the high polarity aqueous medium. More-
over, there may be an entropic effect that destabilizes the
H bonds between water and an unfolded polypeptide rela-
tive to intraprotein H bonds: The water molecules that are
H bonded to a polypeptide are likely to be more position-
ally and orientationally constrained (ordered) than those
that are H bonded to only other water molecules, thus fa-
voring the formation of intraprotein H bonds.These effects
may very well account for the observation that the muta-
genic removal of an H bond from a protein generally re-
duces the protein’s stability by ⫺2 to 8 kJ ⴢ mol
⫺1
.
Despite their low stability, a protein’s hydrogen bonds
provide a structural basis for its native folding pattern: If a
protein folded in a way that prevented some of its internal
H bonds from forming, their free energy would be lost and
such conformations would be less stable than those that are
fully H bonded. In fact, the formation of ␣ helices and
 sheets efficiently satisfies the polypeptide backbone’s
H bonding requirements.This argument also applies to the
van der Waals forces discussed in the previous section.
b. Most Hydrogen Bonds in Proteins Are Local
How can as complex a molecule as a protein fold so as to
make nearly all of its potential H bonds? The answer to this
question was revealed by a survey of the H bonds in high
resolution protein X-ray structures by Ken Dill and George
Rose: Most of the H bonds in a protein are local, that is, they
involve donors and acceptors that are close together in se-
quence and hence can readily find their H bonding mates.
1. On average, 68% of the H bonds in proteins are be-
tween backbone atoms. Of these, ⬃1/3 form n S n ⫺ 4 H
bonds (as in ideal ␣ helices), ⬃1/3 form n S n ⫺ 3 H bonds
(as in reverse turns and ideal 3
10
helices), and ⬃1/3 are be-
tween paired strands in  sheets. In fact, only ⬃5% of the
H bonds between backbone atoms are not wholly within a
helix, sheet, or turn.
2. Hydrogen bonds between side chains and backbones
are clustered at helix-capping positions. In an ␣ helix, the
first four N¬H groups and the last four groups can-
not form H bonds within the helix (which accounts for half
the potential H bonds involving backbone atoms in an ␣ he-
lix of 12 residues, the average length of ␣ helices).These po-
tential H bonds are often made with nearby side chains. In
particular,⬃1/2 of the N-terminal N¬H groups of ␣ helices
form H bonds with polar side chains that are 1 to 3 residues
distant, and ⬃1/3 of their C-terminal groups form H
bonds with polar side chains that are 2 to 5 residues distant.
3. Over half the H bonds between side chains are be-
tween charged residues (i.e., they form salt bridges) and
are therefore located on protein surfaces between and
within surface loops (e.g., Fig. 8-57). However, ⬃85% of the
remaining side chain–side chain H bonds are between side
chains that are 1 to 5 residues apart. Hence with the excep-
tion of those in salt bridges, side chain–side chain H bonds
also tend to be local.
C “ O
C “ O
C. Hydrophobic Forces
The hydrophobic effect is the name given to those influences
that cause nonpolar substances to minimize their contacts
with water and amphipathic molecules, such as soaps and de-
tergents, to form micelles in aqueous solutions (Section 2-
1Ba). Since native proteins form a sort of intramolecular
micelle in which their nonpolar side chains are largely out of
contact with the aqueous solvent, hydrophobic interactions
must be an important determinant of protein structures.
The hydrophobic effect derives from the special proper-
ties of water as a solvent,only one of which is its high dielec-
tric constant. In fact, other polar solvents, such as dimethyl
sulfoxide (DMSO) and N,N-dimethylformamide (DMF),
tend to denature proteins. The thermodynamic data of
Table 8-5 provide considerable insight as to the origin of the
hydrophobic effect because the transfer of a hydrocarbon
from water to a nonpolar solvent resembles the transfer of
a nonpolar side chain from the exterior of a protein in aque-
ous solution to its interior. The isothermal Gibbs free en-
ergy changes (⌬G ⫽⌬H ⫺ T ⌬S) for the transfer of a hydro-
carbon from an aqueous solution to a nonpolar solvent is
negative in all cases, which indicates, as we know to be the
case, that such transfers are spontaneous processes (oil and
water don’t mix). What is perhaps unexpected is that these
transfer processes are endothermic (positive ⌬H) for
aliphatic compounds and athermic (⌬H ⫽ 0) for aro-
matic compounds; that is, it is enthalpically more or equally
favorable for nonpolar molecules to dissolve in water than in
nonpolar media. In contrast, the entropy component of the
unitary free energy change, ⫺T ⌬S
u
(see footnote a to Table
8-5), is large and negative in all cases. Evidently, the transfer
of a hydrocarbon from an aqueous medium to a nonpolar
medium is entropically driven. The same is true of the trans-
fer of a nonpolar protein group from an aqueous environ-
ment to the protein’s nonpolar interior.
What is the physical mechanism whereby nonpolar enti-
ties are excluded from aqueous solutions? Recall that en-
tropy is a measure of the order of a system; it decreases
with increasing order (Section 3-2). Thus the decrease in
entropy when a nonpolar molecule or side chain is solvated
by water (the reverse of the foregoing process) must be
due to an ordering process. This is an experimental obser-
vation, not a theoretical conclusion.The magnitudes of the
entropy changes are too large to be attributed only to
changes in the conformations of the hydrocarbons; rather,
as Henry Frank and Marjorie Evans pointed out in 1945,
these entropy changes mainly arise from some sort of order-
ing of the water structure.
Liquid water has a highly ordered and extensively H
bonded structure (Section 2-1A). The insinuation of a non-
polar group into this structure disrupts it:A nonpolar group
can neither accept nor donate H bonds, so the water mole-
cules at the surface of the cavity occupied by the nonpolar
group cannot H bond to other molecules in their usual fash-
ion. In order to recover the lost H bonding energy, these
surface waters must orient themselves so as to form an H
bonded network enclosing the cavity (Fig. 8-59).This orien-
tation constitutes an ordering of the water structure, since
the number of ways that water molecules can form H bonds
262 Chapter 8. Three-Dimensional Structures of Proteins
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 262
about the surface of a nonpolar group is less than the num-
ber of ways that they can H bond in bulk water.
Unfortunately, the complexity of liquid water’s basic
structure (Section 2-1Ac) has not yet allowed a detailed
structural description of this ordering process. One model
that has been proposed is that water forms quasi-
crystalline H bonded cages about the nonpolar groups sim-
ilar to those of clathrates (Fig. 8-60).The magnitudes of the
entropy changes that result when nonpolar substances are
dissolved in water, however, indicate that the resulting water
structures can only be slightly more ordered than bulk water.
They also must be quite different from that of ordinary ice,
Section 8-4. Protein Stability 263
Table 8-5 Thermodynamic Changes for Transferring Hydrocarbons from Water to Nonpolar Solvents at 25°C
a
Process ⌬H (kJ ⴢ mol
⫺1
) ⫺T ⌬S
u
(kJ ⴢ mol
⫺1
) ⌬G
u
(kJ ⴢ mol
⫺1
)
11.7 ⫺22.6 ⫺10.9
10.5 ⫺22.6 ⫺12.1
9.2 ⫺25.1 ⫺15.9
6.7 ⫺18.8 ⫺12.1
0.8 ⫺8.8 ⫺8.0
0.0 ⫺17.2 ⫺17.2
0.0 ⫺20.0 ⫺20.0Toluene in H
2
O Δ liquid toluene
b
Benzene in H
2
O Δ liquid benzene
b
C
2
H
2
in H
2
O Δ C
2
H
2
in benzene
C
2
H
4
in H
2
O Δ C
2
H
4
in benzene
C
2
H
6
in H
2
O Δ C
2
H
6
in benzene
CH
4
in H
2
O Δ CH
4
in CCl
4
CH
4
in H
2
O Δ CH
4
in C
6
H
6
a
⌬G
u
, the unitary Gibbs free energy change, is the Gibbs free energy change, ⌬G, corrected for its concentration dependence so that it reflects only the
inherent properties of the substance in question and its interaction with solvent.This relationship, according to Equation [3.13], is
where [A
i
] and [A
f
] are the initial and final concentrations of the substance under consideration, respectively, and n is the number of moles of that
substance. Since the second term in this equation is a purely entropic term (concentrating a substance increases its order), ⌬S
u
, the unitary entropy
change, is expressed
b
Data measured at 18°C.
Source: Kauzmann,W., Adv. Protein Chem. 14, 39 (1959).
¢S
u
⫽ ¢S ⫹ nR ln
[A
f
]
[A
i
]
¢G
u
⫽ ¢G ⫺ nRT ln
[A
f
]
[A
i
]
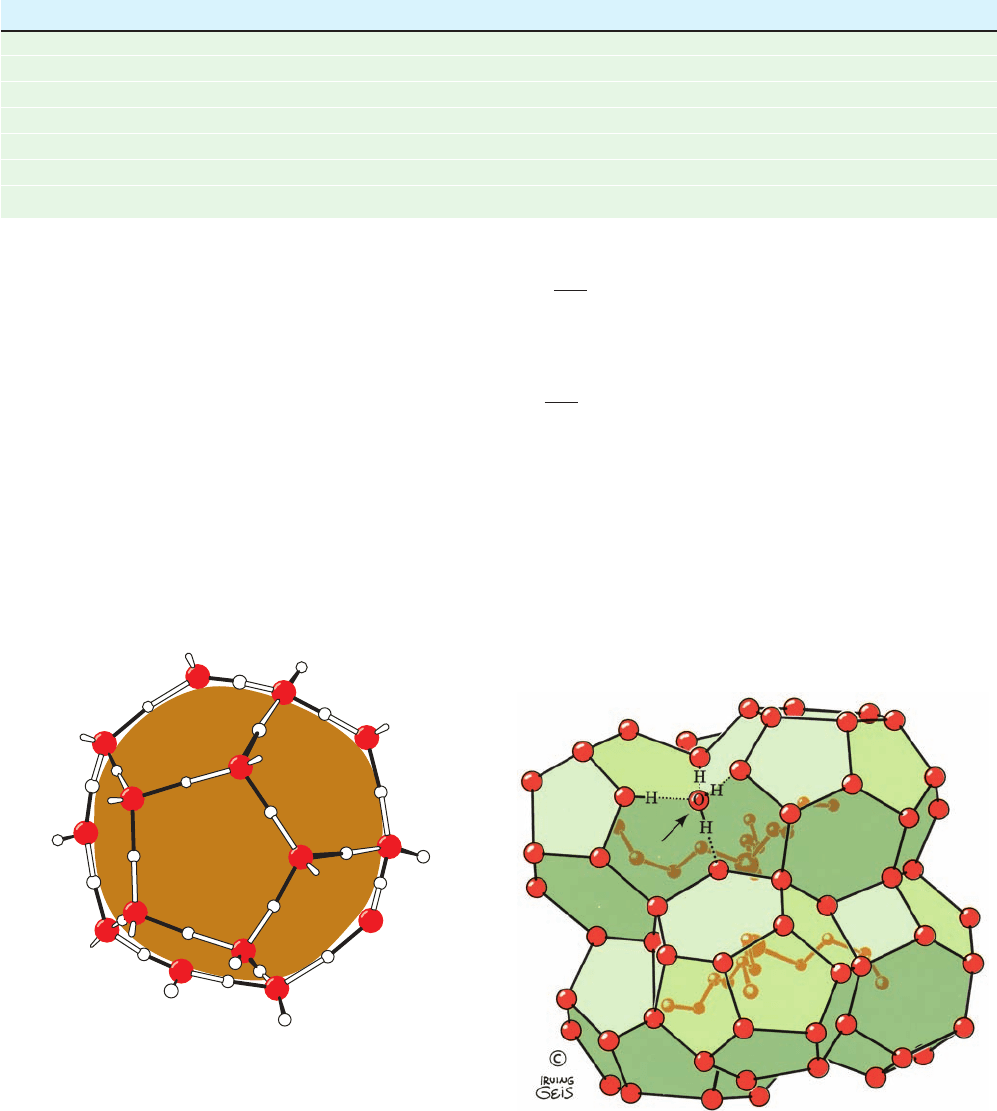
Figure 8-59 The orientational preference of water molecules
next to a nonpolar solute. In order to maximize their H bonding
energy, these water molecules tend to straddle the inert solute
such that, for relatively small solutes, two or three of their
tetrahedral directions are tangential to its surface. This permits
them to form H bonds (black) with neighboring water molecules
lining the nonpolar surface. However, for larger (flatter) nonpolar
solutes, the adjacent water molecules are each geometrically
limited to participating in no more than three H bonds. In either
case, the ordering of water molecules extends several layers of
water molecules beyond the first hydration shell of the nonpolar
solute. [Illustration, Irving Geis. Image from the Irving Geis
Collection, Howard Hughes Medical Institute. Reprinted with
permission.]
Figure 8-60 Structure of the clathrate (n-C
4
H
9
)
3
S
ⴙ
F
ⴚ
⭈ 23H
2
O.
Clathrates are crystalline complexes of nonpolar compounds
with water (usually formed at low temperatures and high pressures)
in which the nonpolar molecules are enclosed, as shown, by a
polyhedral cage of tetrahedrally H bonded water molecules
(here represented by only their oxygen atoms).The H bonding
interactions of one such water molecule (arrow) are shown in
detail. [Illustration, Irving Geis. Image from the Irving Geis
Collection, Howard Hughes Medical Institute. Reprinted with
permission.]
Nonpolar
solute
JWCL281_c08_221-277.qxd 8/10/10 11:48 AM Page 263
because, for instance, the solvation of nonpolar groups by
water causes a large decrease in water volume (e.g., the
transfer of CH
4
from hexane to water shrinks the water so-
lution by 22.7 mL ⴢ mol
⫺1
of CH
4
), whereas the freezing of
water results in a 1.6-mL ⴢ mol
⫺1
expansion.
The unfavorable free energy of hydration of a nonpolar
substance caused by its ordering of the surrounding water
molecules has the net result that the nonpolar substance is
excluded from the aqueous phase. This is because the sur-
face area of a cavity containing an aggregate of nonpolar
molecules is less than the sum of the surface areas of the
cavities that each of these molecules would individually oc-
cupy.The aggregation of the nonpolar groups thereby min-
imizes the surface area of the cavity and therefore the en-
tropy loss of the entire system. In a sense, the nonpolar
groups are squeezed out of the aqueous phase by the hy-
drophobic interactions. Thermodynamic measurements in-
dicate that the free energy change of removing a ¬CH
2
¬
group from an aqueous solution is about ⫺3 kJ ⴢ mol
⫺1
.Al-
though this is a relatively small amount of free energy, in
molecular assemblies involving large numbers of nonpolar
contacts, hydrophobic interactions are a potent force.
Walter Kauzmann pointed out in 1958 that hydrophobic
forces are a major influence in causing proteins to fold into
their native conformations. Figure 8-61 indicates that the
amino acid side chain hydropathies (indexes of combined
hydrophobic and hydrophilic tendencies; Table 8-6) are, in
fact, good predictors of which portions of a polypeptide
chain are inside a protein, out of contact with the aqueous
solvent, and which portions are outside, in contact with the
aqueous solvent. In proteins, the effects of hydrophobic
forces are often termed hydrophobic bonding, presumably
to indicate the specific nature of protein folding under the
influence of the hydrophobic effect. You should keep in
mind, however, that hydrophobic bonding does not gener-
ate the directionally specific interactions usually associated
with the term “bond.”
D. Disulfide Bonds
Since disulfide bonds form as a protein folds to its native
conformation (Section 9-1A), they function to stabilize its
three-dimensional structure.The relatively reducing chemi-
cal character of the cytoplasm, however, greatly diminishes
the stability of intracellular disulfide bonds. In fact, almost
all proteins with disulfide bonds are secreted to more oxi-
dized extracellular destinations, where their disulfide bonds
264 Chapter 8. Three-Dimensional Structures of Proteins
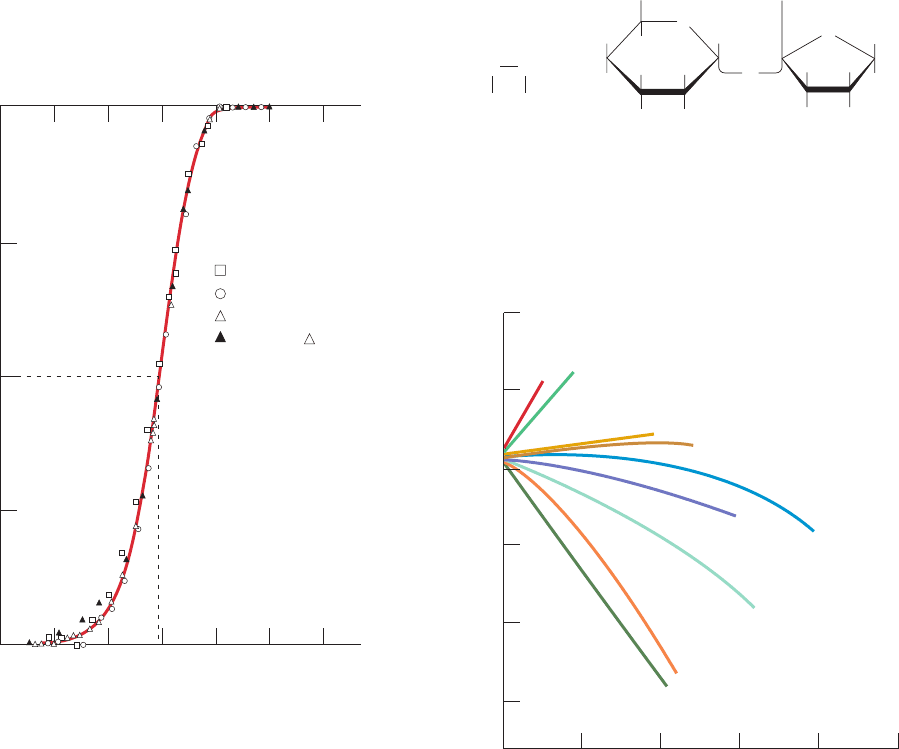
Figure 8-61 Hydropathic index plot for bovine
chymotrypsinogen. The sum of the hydropathies of nine
consecutive residues (see Table 8-6) are plotted versus the residue
sequence number.A large positive hydropathic index is indicative
of a hydrophobic region of the polypeptide chain, whereas a
0
40
20
0
–20
–40
20 40 60 80 100 120 140
Residue number
Hydrophobic
Hydrophilic
160 180 200 220 240
Hydropathic index
Table 8-6 Hydropathy Scale for Amino Acid Side Chains
Side Chain Hydropathy
Ile 4.5
Val 4.2
Leu 3.8
Phe 2.8
Cys 2.5
Met 1.9
Ala 1.8
Gly ⫺0.4
Thr ⫺0.7
Ser ⫺0.8
Tr p ⫺0.9
Ty r ⫺1.3
Pro ⫺1.6
His ⫺3.2
Glu ⫺3.5
Gln ⫺3.5
Asp ⫺3.5
Asn ⫺3.5
Lys ⫺3.9
Arg ⫺4.5
Source: Kyte, J. and Doolitle, R.F., J. Mol. Biol. 157, 110 (1982).
large negative value is indicative of a hydrophilic region.The
bars above the midpoint line denote the protein’s interior
regions, as determined by X-ray crystallography, and the bars
below the midpoint line indicate the protein’s exterior regions.
[After Kyte, J. and Doolittle, R.F., J. Mol. Biol. 157, 111 (1982).]
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 264
are effective in stabilizing protein structures [secreted pro-
teins fold to their native conformations—and hence form
their disulfide bonds—in the endoplasmic reticulum (Sec-
tion 12-4Ba), which, unlike other cellular compartments,
has an oxidizing environment]. Apparently, the relative
“hostility” of extracellular environments toward proteins
(e.g., uncontrolled temperatures and pH’s) requires the ad-
ditional structural stability conferred by disulfide bonds.
E. Protein Denaturation
The low conformational stabilities of native proteins make
them easily susceptible to denaturation by altering the bal-
ance of the weak nonbonding forces that maintain the na-
tive conformation.When a protein in solution is heated, its
conformationally sensitive properties, such as optical rota-
tion (Section 4-2A), viscosity, and UV absorption, change
abruptly over a narrow temperature range (e.g., Fig. 8-62).
Such a nearly discontinuous change indicates that the native
protein structure unfolds in a cooperative manner:Any par-
tial unfolding of the structure destabilizes the remaining
structure,which must simultaneously collapse to the random
coil. The temperature at the midpoint of this process is
known as the protein’s melting temperature, T
m
, in analogy
with the melting of a solid. Most proteins have T
m
values
well below 100°C. Recall that nucleic acids likewise have
characteristic T
m
’s (Section 5-3Ca).
In addition to high temperatures, proteins are dena-
tured by a variety of other conditions and substances:
1. pH variations alter the ionization states of amino
acid side chains (Table 4-1), which changes protein charge
distributions and H bonding requirements.
2. Detergents, some of which significantly perturb pro-
tein structures at concentrations as low as 10
⫺6
M,hy-
drophobically associate with the nonpolar residues of a
protein, thereby interfering with the hydrophobic interac-
tions responsible for the protein’s native structure.
3. High concentrations of water-soluble organic sub-
stances, such as aliphatic alcohols, interfere with the hy-
drophobic forces stabilizing protein structures through
their own hydrophobic interactions with water. Organic
substances with several hydroxyl groups, such as ethylene
glycol or sucrose,
however, are relatively poor denaturants because their H
bonding ability renders them less disruptive of water structure.
The influence of salts is more variable. Figure 8-63
shows the effects of a number of salts on the T
m
of bovine
SucroseEthylene glycol
CH
2
OH
CH
2
OH
H OH
HO
OH
H
H
H
H
HO
H
HOH
CH
2
OH
H
O
O
O
H
2
CCH
2
HO OH
Section 8-4. Protein Stability 265
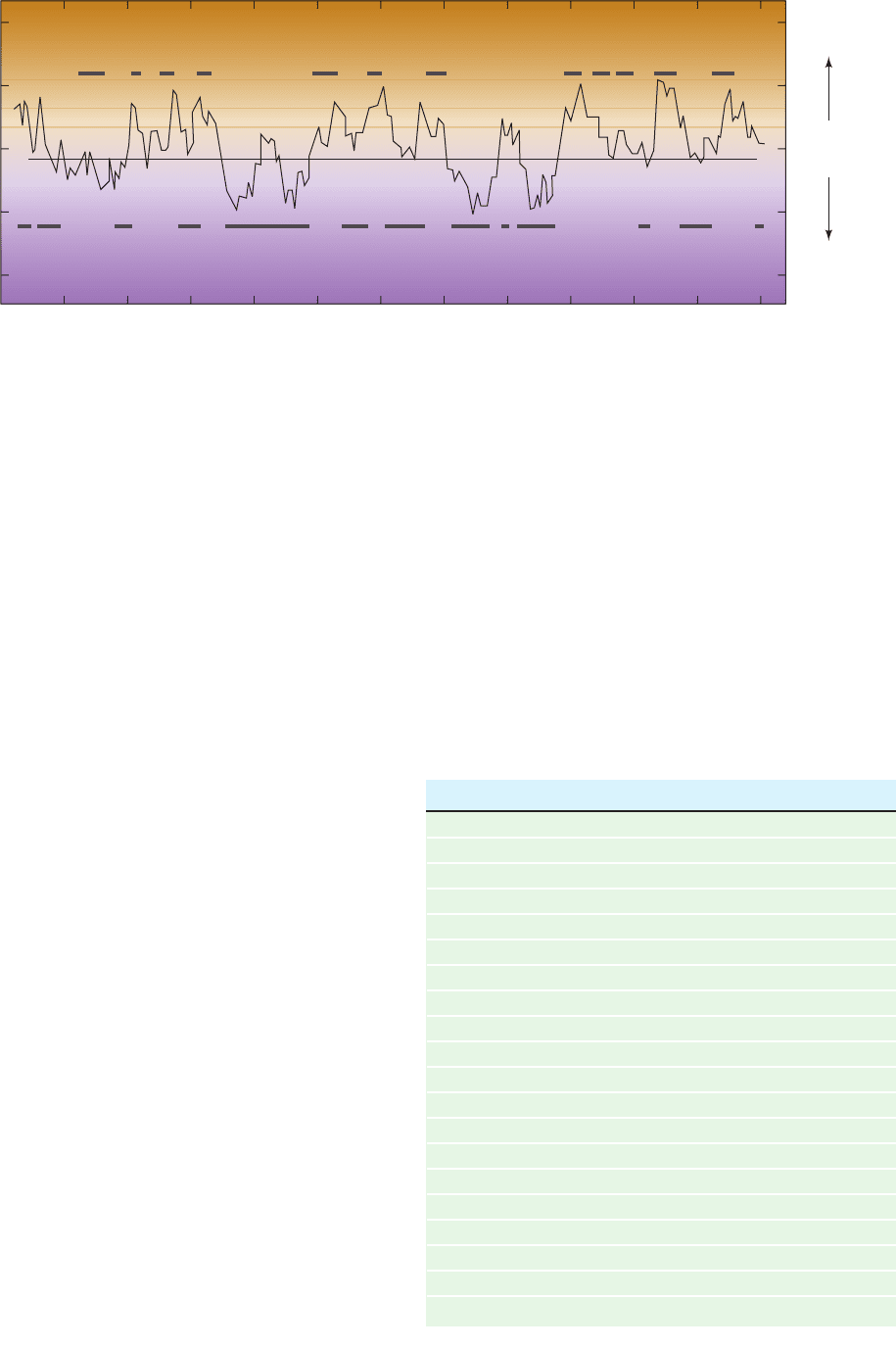
Figure 8-62 Protein denaturation. The heat-induced
denaturation of bovine pancreatic ribonuclease A (RNase A) in an
HCl–KCl solvent at pH 2.1 and 0.019 ionic strength was monitored
by several conformationally sensitive techniques.The curve is drawn
only through the points ⌬.The melting temperature, T
m
, is defined
as the temperature at the midpoint of the transition. Compare the
shape of this melting curve with that of duplex DNA (Fig. 5-16).
[After Ginsburg,A. and Carroll,W.R., Biochemistry 4, 2169 (1965).]
0 102030405060
0.25
0.50
0.75
1.00
0
Fractional change
T (°C)
Intrinsic viscosity
Specific rotation at 365 n
Molar absorbance at 287
Repeat of after cooling
for ~16 hours
T
m
01234 5
30
40
50
60
70
80
KH
2
PO
4
(pH6.6)
(NH
4
)
2
SO
4
KCl
NaCl
LiCl
NaBr
LiBr
CaCl
2
KSCN
Concentration (M)
T
m
(°C)
Figure 8-63 Melting temperature of RNase A as a function of
the concentrations of various salts. All solutions also contained
0.15M KCl and 0.013M sodium cacodylate buffer, pH 7. [After
von Hippel, P.J. and Wong, K.Y., J. Biol. Chem. 10, 3913 (1965).]
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 265
pancreatic ribonuclease A (RNase A). Some salts, such as
(NH
4
)
2
SO
4
and KH
2
PO
4
, stabilize the native protein struc-
ture (raise its T
m
); others, such as KCl and NaCl, have little
effect; and yet others, such as KSCN and LiBr, destabilize
it.The order of effectiveness of the various ions in stabiliz-
ing a protein, which is largely independent of the identity
of the protein, parallels their capacity to salt out proteins
(Section 6-2A). This order is known as the Hofmeister
series:
Anions:
Cations:
The ions in the Hofmeister series that tend to denature
proteins, I
⫺
, , SCN
⫺
,Li
⫹
,Mg
2⫹
,Ca
2⫹
, and Ba
2⫹
,are
said to be chaotropic. This list should also include the
guanidinium ion (Gu
⫹
) and the nonionic urea, which, in
concentrations in the range 5 to 10 M, are the most com-
monly used protein denaturants. The effect of the various
ions on proteins is largely cumulative: GuSCN is a much
more potent denaturant than the often used GuCl, whereas
Gu
2
SO
4
stabilizes protein structures.
Chaotropic agents increase the solubility of nonpolar
substances in water. Consequently, their effectiveness as
denaturing agents stems from their ability to disrupt hy-
drophobic interactions, although the manner in which they
do so is not well understood. Conversely, those substances
listed that stabilize proteins strengthen hydrophobic forces,
thus increasing the tendency of water to expel proteins.
This accounts for the correlation between the abilities of an
ion to stabilize proteins and to salt them out.
F. Explaining the Stability of Thermostable Proteins
Certain species of bacteria known as hyperthermophiles
grow at temperatures near 100°C (they live in such places
as hot springs and submarine hydrothermal vents, with the
most extreme, an Fe(III)-reducing archaeon, growing at
121°C and remaining viable as high as 130°C).These organ-
isms have many of the same metabolic pathways as do
mesophiles (organisms that grow at “normal” tempera-
tures). Yet, most mesophilic proteins denature at the tem-
peratures at which hyperthermophiles thrive. What is the
structural basis for the thermostability of hyperther-
mophilic proteins?
The difference in the thermal stabilities of the corre-
sponding (hyper)thermophilic and mesophilic proteins
does not exceed ⬃100 kJ ⴢ mol
⫺1
, the equivalent of a few
noncovalent interactions. This is probably why compar-
isons of the X-ray structures of hyperthermophilic en-
zymes with their mesophilic counterparts have failed to re-
veal any striking differences between them.These proteins
exhibit some variations in secondary structure but no more
so than is often the case for homologous proteins from dis-
tantly related mesophiles. However, several of these ther-
mostable enzymes have a superabundance of salt bridges
on their surfaces, many of which are arranged in extensive
ClO
⫺
4
⬎ Ca
2⫹
⬎ Ba
2⫹
NH
⫹
4
, Cs
⫹
, K
⫹
, Na
⫹
7 Li
⫹
7 Mg
2⫹
⬎ Br
⫺
⬎ I
⫺
⬎ ClO
⫺
4
⬎ SCN
⫺
SO
2⫺
4
⬎ H
2
PO
⫺
4
⬎ CH
3
COO
⫺
⬎ Cl
⫺
networks. Indeed, one such network from Pyrococcus fu-
riosus glutamate dehydrogenase consists of 18 side chains.
The idea that salt bridges can stabilize a protein struc-
ture appears to contradict the conclusion of Section 8-4Aa
that ion pairs are, at best, marginally stable. The key to this
apparent paradox is that the salt bridges in thermostable
proteins form networks. Thus, the gain in charge–charge
free energy on associating a third charged group with an
ion pair is comparable to that between the members of this
ion pair, whereas the free energy lost on desolvating and
immobilizing the third side chain is only about half that lost
in bringing together the first two side chains. The same, of
course, is true for the addition of a fourth, fifth, etc., side
chain to a salt bridge network.
Not all thermostable proteins have such a high inci-
dence of salt bridges. Structural comparisons suggest that
these proteins are stabilized by a combination of small ef-
fects, the most important of which are an increased size in
the protein’s hydrophobic core, an increased size in the in-
terface between its domains and/or subunits, and a more
tightly packed core as evidenced by a reduced surface-to-
volume ratio.
The fact that the proteins of hyperthermophiles and
mesophiles are homologous and carry out much the same
functions indicates that mesophilic proteins are by no
means maximally stable.This, in turn, strongly suggests that
the marginal stability of most proteins under physiological
conditions (averaging ⬃0.4 kJ/mol of amino acid residues)
is an essential property that has arisen through evolutionary
design. Perhaps this marginal stability helps confer the
structural flexibility that many proteins require to carry
out their physiological functions (Section 9-4). Other possi-
bilities are that it may facilitate the elimination of other-
wise stable non-native conformations (Section 9-2C), it
may promote the unfolding of proteins so as to permit their
insertion into or transport through membranes (Section
12-4E), and/or it may expedite their programmed degrada-
tion (Section 32-6).
5 QUATERNARY STRUCTURE
Proteins, because of their multiple polar and nonpolar
groups, stick to almost anything; anything, that is, but other
proteins. This is because the forces of evolution have
arranged the surface groups of proteins so as to prevent
their association under physiological conditions. If this
were not the case, their resulting nonspecific aggregation
would render proteins functionally useless (recall, e.g., the
consequences of sickle-cell anemia; Section 7-3A). In his
pioneering ultracentrifugational studies on proteins, how-
ever, The Svedberg discovered that some proteins are
composed of more than one polypeptide chain. Subsequent
studies established that this is, in fact, true of most proteins,
including nearly all those with molecular masses ⬎100 kD.
Furthermore, these polypeptide subunits associate in a
geometrically specific manner. The spatial arrangement of
these subunits is known as a protein’s quaternary structure
(4° structure).
266 Chapter 8. Three-Dimensional Structures of Proteins
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 266
There are several reasons why multisubunit proteins are
so common. In large assemblies of proteins, such as colla-
gen fibrils, the advantages of subunit construction over the
synthesis of one huge polypeptide chain are analogous to
those of using prefabricated components in constructing a
building: Defects can be repaired by simply replacing the
flawed subunit rather than the entire protein, the site of
subunit manufacture can be different from the site of as-
sembly into the final product, and the only genetic infor-
mation necessary to specify the entire edifice is that speci-
fying its few different self-assembling subunits. In the case
of enzymes, increasing a protein’s size tends to better fix
the three-dimensional positions of the groups forming the
enzyme’s active site. Increasing the size of an enzyme
through the association of identical subunits is more effi-
cient, in this regard, than increasing the length of its
polypeptide chain since each subunit has an active site.
Additionally, in some multimeric enzymes, the active site oc-
curs at the interface between subunits where it is com-
prised of groups from two or more subunits. More impor-
tantly, however, the subunit construction of many enzymes
provides the structural basis for the regulation of their ac-
tivities. Mechanisms for this indispensable function are dis-
cussed in Sections 10-4 and 13-4.
In this section we discuss how the subunits of multisub-
unit proteins associate, what sorts of symmetries they have,
and how their stoichiometries may be determined.
A. Subunit Interactions
A multisubunit protein may consist of identical or non-
identical polypeptide chains. Recall that hemoglobin, for
example, has the subunit composition ␣
2

2
. We shall refer
to proteins with identical subunits as oligomers and to
these identical subunits as protomers. A protomer may
therefore consist of one polypeptide chain or several unlike
polypeptide chains. In this sense, hemoglobin is a dimer
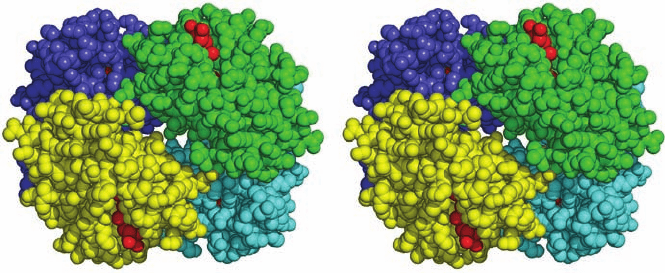
(oligomer of two protomers) of ␣ protomers (Fig. 8-64).
The association of two subunits typically buries 1000 to
2000 Å
2
of surface area (minimally ⬃600 Å
2
) that would
otherwise be exposed to solvent. The resulting contact re-
gions superficially resemble the interiors of single subunit
proteins: They contain closely packed nonpolar side
chains, hydrogen bonds, and in some cases, interchain disul-
fide bonds. However, protein–protein interfaces differ
from subunit interiors in several respects:
1. They tend to have hydrophobicities between those of
protein interiors and exteriors. In particular, the subunit in-
terfaces of proteins that dissociate in vivo have lesser hy-
drophobicities than do permanent interfaces.
2. An average of ⬃77% of intersubunit hydrogen
bonds are between side chains. In contrast, an average of
⬃68% of the hydrogen bonds within subunits are between
backbone atoms. This is mainly because secondary struc-
tural elements are not continued across subunit boundaries
(with the occasional exception of  sheets; see below).
3. Around 56% of protein–protein interfaces contain
salt bridges.Th\ese contribute to the specificity as well as to
the stability of subunit associations.
In addition, there are negligibly few hydrogen bonds and
salt bridges at the edges of the contact regions. Not surpris-
ingly, the residues at protein–protein interfaces are evolu-
tionarily well conserved compared to other surface
residues.
B. Symmetry in Proteins
In the vast majority of oligomeric proteins, the protomers
are symmetrically arranged; that is, the protomers occupy
geometrically equivalent positions in the oligomer. This
implies that each protomer has exhausted its capacity to bind
to other protomers; otherwise, higher oligomers would
form.As a result of this limited binding capacity, protomers
pack about a single point to form a closed shell, a phenom-
enon known as point symmetry. Proteins cannot have in-
version or mirror symmetry, however, because such sym-
metry operations convert chiral
L-residues to D-residues.
Thus, proteins can only have rotational symmetry.
Section 8-5. Quaternary Structure 267
Figure 8-64 The quaternary structure of hemoglobin. The ␣
1
,
␣
2
, 
1
, and 
2
subunits in this stereo, space-filling drawing are
colored yellow, green, blue, and cyan, respectively. Heme groups
are red.The protein is viewed along its molecular 2-fold rotation
axis, which relates the ␣
1

1
protomer to the ␣
2

2
protomer.
Instructions for viewing stereo drawings are given in the
appendix to this chapter. [Based on an X-ray structure by Max
Perutz, MRC Laboratory of Molecular Biology, Cambridge, U.K.
PDBid 2DHB.]
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 267
Various types of rotational symmetry occur in proteins,
as X-ray crystal structure determinations have shown:
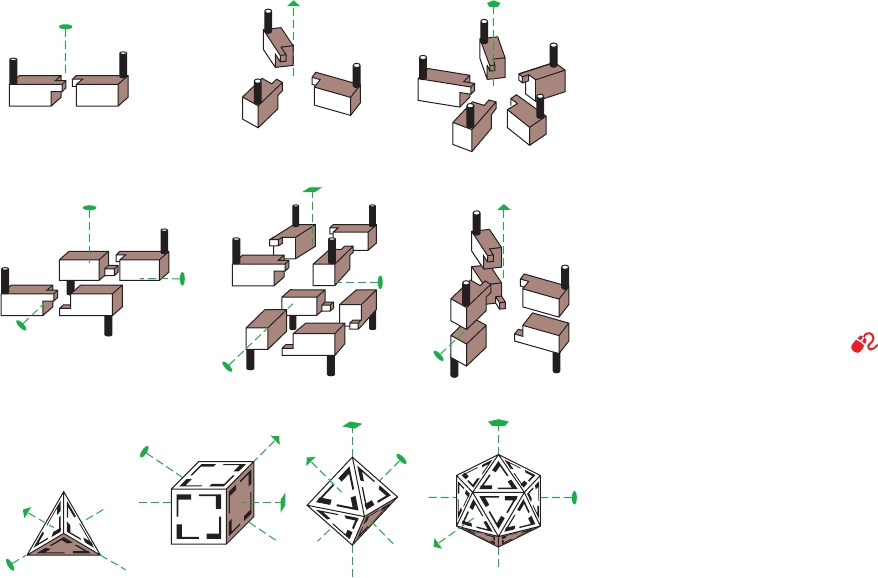
1. Cyclic symmetry
In the simplest type of rotational symmetry, cyclic sym-
metry, subunits are related (brought to coincidence) by a
single axis of rotation (Fig. 8-65a). Objects with 2-, 3-,...,
or n-fold rotational axes are said to have C
2
, C
3
,...,or C
n
symmetry, respectively. An oligomer with C
n
symmetry
consists of n protomers that are related by (360/n)° rota-
tions. C
2
symmetry is the most common symmetry in pro-
teins; higher cyclic symmetries are relatively rare.
A common mode of association between protomers
related by a twofold rotation axis is the continuation of a
 sheet across subunit boundaries. In such cases, the 2-fold
axis is perpendicular to the  sheet so that two symmetry
equivalent  strands hydrogen bond in an antiparallel fash-
ion. In this manner, the sandwich of two 4-stranded  sheets
in a protomer of transthyretin (also known as prealbumin)
is extended across a 2-fold axis to form a sandwich of two
8-stranded  sheets (Fig. 8-66). Hemoglobin’s two ␣ pro-
tomers are also related by C
2
symmetry (Fig. 8-64).
2. Dihedral symmetry
Dihedral symmetry (D
n
), a more complicated type of
rotational symmetry, is generated when an n-fold rotation
axis and a 2-fold rotation axis intersect at right angles
(Fig. 8-65b). An oligomer with (D
n
) symmetry consists of
2n protomers. D
2
symmetry is, by far, the most common
type of dihedral symmetry in proteins.
Hemoglobin’s ␣ and  subunits have such similar
structures that, in the hemoglobin ␣
2

2
tetramer, they are
related by pseudo-2-fold rotational axes that are perpen-
dicular to the tetramer’s exact 2-fold axis (lie in the plane
of Fig. 8-64; Section 10-2B). Hence the tetramer is said to
be pseudosymmetric and have pseudo-D
2
symmetry. The
X-ray structure of glutamine synthetase reveals that this
enzyme consists of 12 identical subunits that are related
by D
6
symmetry (Fig. 8-67).
Under the proper conditions, many oligomers with D
n
symmetry dissociate into two oligomers, each with C
n
sym-
metry (and which are related by the 2-fold rotation axes in
the D
n
oligomer).These, in turn, dissociate to their compo-
nent protomers under more stringent denaturing condi-
tions.
3. Other rotational symmetries
The only other types of rotationally symmetric objects
are those that have the rotational symmetries of a tetrahe-
dron (T ), a cube or octahedron (O), or a dodecahedron or
icosahedron (I ), and hence have 12, 24, and 60 equivalent
positions, respectively (Fig. 8-65c). Certain multienzyme
complexes are based on octahedral symmetry (Section
21-2A), whereas others have icosahedral symmetry (Sec-
tion 21-2Aa). The protein coats of the so-called spherical
viruses also have icosahedral symmetry (Section 33-2A).
268 Chapter 8. Three-Dimensional Structures of Proteins
(a) Cyclic symmetries
C
2
(b) Dihedral symmetries
D
2
Tetrahedral
symmetry
Octahedral (cubic)
symmetry
Icosahedral
symmetry
T
OI
C
3
D
4
C
5
D
3
(c)
Figure 8-65 Some possible symmetries of
proteins with identical protomers. The lenticular
shape, the triangle, the square, and the pentagon at
the ends of the dashed lines indicate, respectively,
the unique 2-fold, 3-fold, 4-fold, and 5-fold
rotational axes of the objects shown. (a)
Assemblies with the cyclic symmetries C
2
, C
3
, and
C
5
.(b) Assemblies with the dihedral symmetries
D
2
, D
4
, and D
3
. In these objects, a 2-fold axis is
perpendicular to the vertical 2-, 4-, and 3-fold axes.
(c) Assemblies with T, O, and I symmetry. Note
that the tetrahedron has some but not all of the
symmetry elements of the cube, and that the cube
and the octahedron have the same symmetry.
[Illustration, Irving Geis. Image from the Irving
Geis Collection, Howard Hughes Medical Insti-
tute. Reprinted with permission.]
See the
Animated Figures
JWCL281_c08_221-277.qxd 8/10/10 11:48 AM Page 268
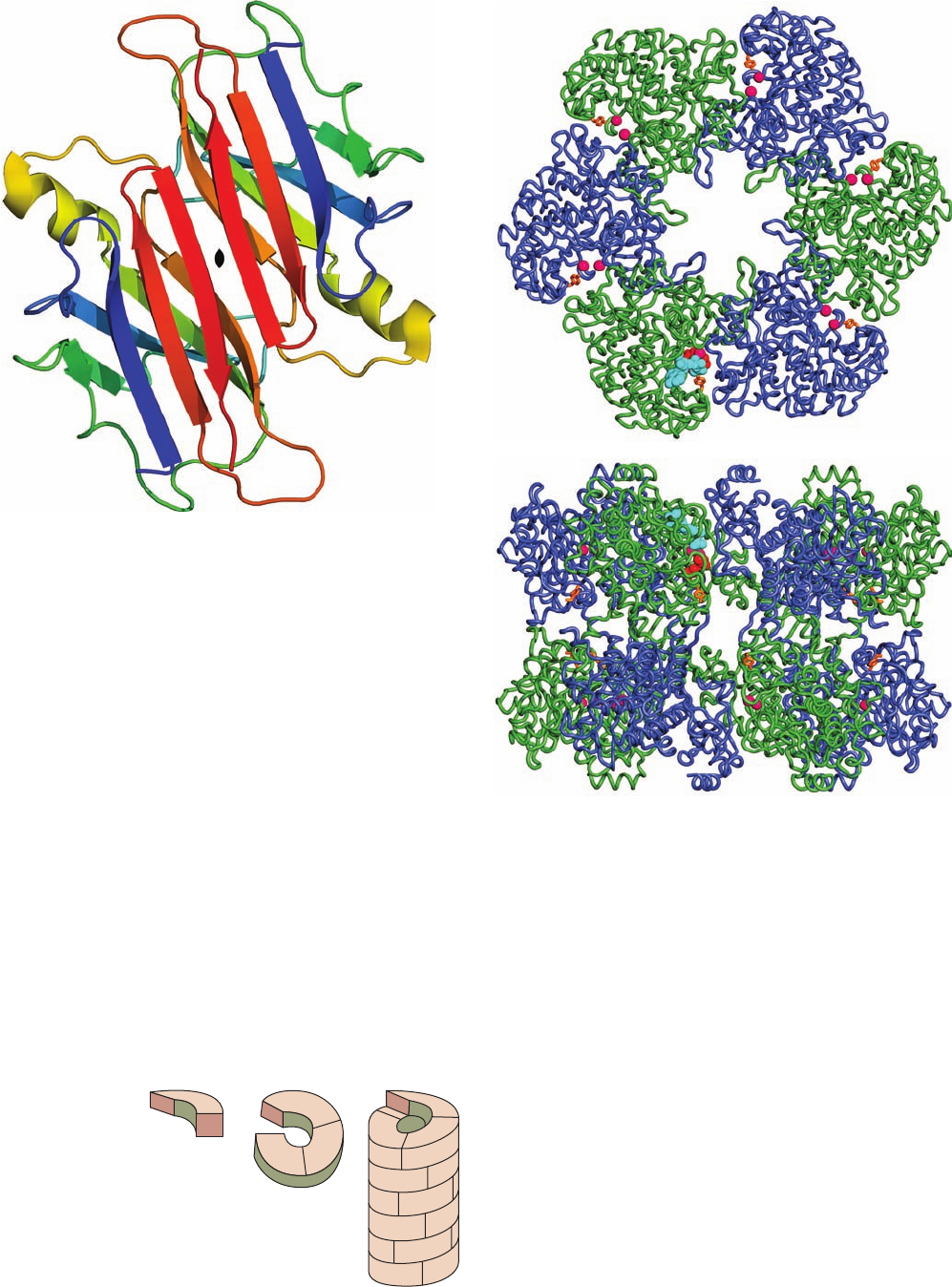
a. Helical Symmetry
Some protein oligomers have helical symmetry (Fig.8-68).
The chemically identical subunits in a helix are not strictly
equivalent because, for instance, those at the ends of the
helix have a different environment than those in the mid-
dle. Nevertheless, the surroundings of all subunits in a long
helix, except those near its ends, are sufficiently similar that
the subunits are said to be quasi-equivalent. The subunits
of many structural proteins, for example, those of actin
(Section 35-3Ad) and tubulin (Section 35-3Gc), assemble
into fibers with helical symmetry.
Section 8-5. Quaternary Structure 269
Figure 8-66 A dimer of transthyretin as viewed down its
2-fold axis (black lenticular symbol). Each protomer, which is
colored in rainbow order from its N-terminus (blue) to its
C-terminus (red), consists of a  barrel (really a  sandwich)
containing two Greek keys (Fig. 8-50a). Note how both of its 
sheets are continued in an antiparallel fashion in the symmetry-
related protomer to form a sandwich of two 8-stranded  sheets.
Two of these dimers associate back to back in the native protein
to form a tetramer with D
2
symmetry. [Based on an X-ray
structure by Colin Blake, Oxford University, U.K. PDBid 2PAB.]
Figure 8-67 X-ray structure of glutamine synthetase from
Salmonella typhimurium. The enzyme consists of 12 identical
subunits, here drawn in worm form, arranged with D
6
symmetry.
(a) View down the 6-fold axis of symmetry showing only the six
subunits of the upper ring in alternating blue and green.The
subunits of the lower ring are roughly directly below those of the
upper ring.The protein, including its side chains (not shown), has
a diameter of 143 Å.The six active sites shown are marked by
pairs of bound Mn
2⫹
ions (magenta spheres).Also drawn in one
active site are ADP (cyan) and the inhibitor phosphinothricin
(red). (b) Side view along one of the protein’s 2-fold axes
showing only its eight nearest subunits.The molecule extends 103
Å along the 6-fold axis, which is vertical in this view. [Based on
an X-ray structure by David Eisenberg, UCLA. PDBid 1FPY.]
(a)
(b)
Subunit
Helix segment
Helix
Figure 8-68 A helical structure composed of a single kind of
subunit.
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 269
b. Obtaining the Atomic Coordinates of a
Biologically Functional Quaternary Structure
Crystals consist of three-dimensional lattices of identi-
cal unit cells (the smallest portion of a crystal lattice that is
repeated by translation) that usually have internal symme-
try. The crystal’s asymmetric unit is the unique portion of
the unit cell from which the entire unit cell can be gener-
ated through the operation of its symmetry elements. In a
crystal of a symmetrical protein, if one or more of protein’s
symmetry axes are coincident with unit cell’s symmetry
axes, the asymmetric unit would contain a subset of the
protein’s protomers (often only one), which would be re-
lated to the other such subsets by crystallographic symme-
try. Alternatively, the asymmetric units in crystals of many
oligomeric proteins contain one or more entire proteins, in
which case their protomers are said to be related by non-
crystallographic symmetry.
A Protein Data Bank (PDB) coordinate file for an X-
ray crystal structure contains the atomic coordinates of the
protomers occupying an asymmetric unit. The entire crys-
tal structure can then be generated through the application
of its crystallographic symmetry. Thus, a symmetric pro-
tein’s PDB coordinate file might contain the coordinates of
only one of its several symmetry-related protomers. More-
over, in some cases, the intermolecular contacts in a crystal
may sufficiently resemble the contacts between the pro-
tomers in an oligomer so that its quaternary structure may
be ambiguous.
To alleviate these difficulties, computerized procedures
have been devised to generate the coordinates of the most
probable biologically functional molecule based on several
criteria, including maximizing the solvent-accessible sur-
face area that is buried on forming the oligomer. The
coordinates of the most probable quaternary structures
of macromolecules whose structures have been deter-
mined by X-ray crystallography are publicly available at
http://www.ebi.ac.uk/msd-srv/prot_int/pistart.html. The bi-
ological unit may also be viewed directly from the corre-
sponding PDB Structure Summary page, although note
that the two algorithms do not always agree.
C. Determination of Subunit Composition
In the absence of an X-ray or NMR structure, the number
of different types of subunits in an oligomeric protein may
be determined by end group analysis (Section 7-1A). In
principle, the subunit composition of a protein may be de-
termined by comparing its molecular mass with those of its
component subunits. In practice, however, experimental
difficulties, such as the partial dissociation of a supposedly
intact protein and uncertainties in molecular mass determi-
nations, often provide erroneous results.
a. Cross-Linking Agents Stabilize Oligomers
A method for 4° structure analysis, which is especially
useful for oligomeric proteins that decompose easily, em-
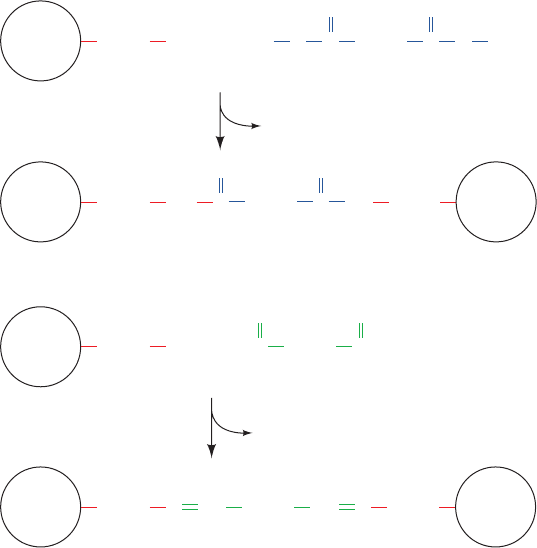
ploys cross-linking agents such as dimethylsuberimidate or
glutaraldehyde (Fig. 8-69). If carried out at sufficiently low
protein concentrations to eliminate intermolecular reac-
tions, cross-linking reactions will covalently join only the
270 Chapter 8. Three-Dimensional Structures of Proteins
Figure 8-69 Cross-linking agents. Dimethylsuberimidate and glutaraldehyde are bifunctional
reagents that react to covalently cross-link two Lys residues.
(CH
2
)
4
NH
2
2CH
3
OH
H
3
CO
NH
C (CH
2
)
6
(CH
2
)
4
NH (CH
2
)
4
NH
(CH
2
)
4
NH
2
(CH
2
)
4
(CH
2
)
4
(CH
2
)
3
CHNN
⫹
CO
NH
2
C (CH
2
)
6
C
CH
3
HC
CH
(CH
2
)
3
⫹
O
O
CH
⫹
NH
2
⫹
2
2 Subunit
Subunit
Subunit Subunit
Subunit
Subunit
Lys Dimethylsuberimidate
Lys Glutaraldehyde
2H
2
O
NH
JWCL281_c08_221-277.qxd 2/23/10 1:59 PM Page 270
