Swaminathan N., Bray K.N.C. (eds.) Turbulent Premixed Flames
Подождите немного. Документ загружается.

4.1 Application of Lean Flames in Internal Combustion Engines 303
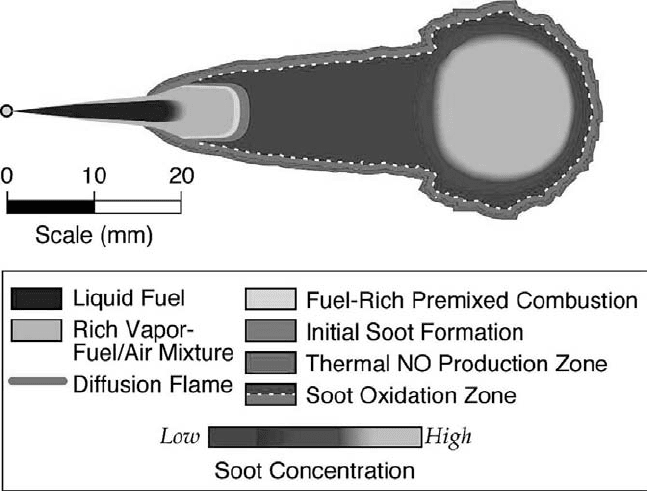
Figure 4.49. A schematic of the conceptual model of conventional diesel combustion with
additional features (fuel-rich premixed flame, soot formation, soot oxidation, and NO forma-
tion zones) that are indicated by the data but have not yet been verified (J. E. Dec, personal
communication and [161]). (See colour plate.)
The result is, however, a surprising one for two reasons [155]. First, the centreline
decay of the equivalence ratio in a quasi-steady jet, without EGR in the ambient, is
proportional to the reciprocal of distance from the nozzle. Thus expectation might
have been that the equivalence ratios should be far in excess of unity in the region
up to about 30 mm; and this expectation would have been further reinforced by the
presence of EGR in the ambient, because mixtures in such a jet should be richer
still because of the reduced oxygen concentration because of EGR in the ambient.
Nevertheless, shortly after the end of injection, the jet has equivalence ratios near
unity in the same region. Second, it is likely that the amount of entrained ambient gas
is similar to that of a conventional jet, because at a given downstream location the
cross-sectional area is comparable. However, shortly after the end of injection, the
equivalence ratio becomes much lower, which implies that the mixing processes after
the end of injection must be more rapid than those occurring during injection. In
conclusion, in a quasi-impulsively stopped jet, the richest parts are far downstream
and not upstream as in a quasi-steady jet. Thus the lean equivalence ratios near
the injector after the end of injection may lead to the UHC emissions because of
incomplete combustion for conditions with long ignition delays.
Gasoline as a CI Fuel. A recent study [165] used high-octane-number fuels, such as
gasoline and ethanol, in the ‘partially premixed combustion’ mode in CI engines.
The premixing is promoted because such fuels are both resistant to auto-ignition,
hence increasing the opportunity for mixing, and highly volatile, with the result
that the emissions of particulates were low. It was found possible to control the
304 Lean Flames in Practice
CA at which 50% MFB and the apparent heat release rate could be low. For some
operating conditions, the gasoline led to very much lower NO
x
emissions than those
of diesel fuel because of the better attainment of lean, premixed conditions. The
rate of pressure rise was controlled by use of double injection, part early and part
near TDC. Initial research was conducted in a 2-L single-cylinder diesel engine
running at 1200 rpm. Kalghatgi et al. [166] recently extended this to a passenger-car
displacement at up to 3000 rpm. At low loads and speeds, low NO
x
emissions are
attainable whereas this is not possible for diesel fuel. At 2000 rpm and between 10
and 12 IMEP, low NO
x
can be maintained with negligible particulate emission by
use of EGR, which is again not possible with diesel fuel. At 3000-rpm 13-bars IMEP,
low NO
x
with negligible smoke was again possible. However, HC and CO emissions
were higher for gasoline and would require removal by an oxidising catalyst. The
gravimetric fuel consumption was similar for both gasoline and diesel fuels at all
operating points. Although there is, as yet, little direct evidence of the extent of lean
combustion in these experiments, it seems likely that it is substantial.
4.1.3 Role of Experiments for Lean-Burn Combustion in IC Engines
After the announcement of Mitsubishi’s first-generation gasoline DI engine in the
mid-1990s, an executive of that company was quoted as saying ‘The break-through
came five years ago when, by using laser technology and high-speed cameras, engi-
neers were able to study exactly what goes on at the moment of ignition’ (cited in
[59]). Related publications suggest that the development of this engine does indeed
seem to have benefited [81] from the development of laser instrumentation that
made planar, rather than point, measurements. Such techniques, in their infancy in
the 1990s, have advanced greatly since then, and recent reviews of the contribution
that can be made by optical instrumentation were published in [167] for gasoline
engines, [168] for diesel engines, and [169] for HCCI engines. Instruments, although
not all providing ‘planar’ information, are available for the measurement of droplet
size, fuel concentration (by tracer LIF) and fuel–air ratio (FAR), of the concen-
tration of each phase in a spray (by exciplex LIF), of the presence of cool flames
and second-stage ignition, of NO concentration and soot particle size and volume
fraction, of thermometry for the soot and gas phases [the latter by Rayleigh, LIF,
or absorption thermometry, or coherent anti-Stokes Raman scattering (CARS), or
microparticle thermographic phosphors) and of the concentration of HCs, H
2
O, CO,
and CO
2
by laser absorption. In addition, the measurement of flow velocity, by point
or planar techniques, is widespread, and soot size and concentration are increasingly
reported by use of laser-induced incandescence (LII) [170].
Some of the instruments in the preceding paragraph have been available, in
one form or another, for well over a decade and are well known and widely used.
The evolution of instruments continues; here we briefly mention some recent de-
velopments that are relevant to research into lean-burn combustion in engines. We
start with the measurement of the atomisation of the liquid fuel. Here planar, as
opposed to point (e.g., phase-Doppler velocimetry), measurements of the liquid
phase have become available for surface impingement [171] and for simultaneous
size and velocity measurements by interferometric laser imaging for droplet sizing
(ILIDS) [172]: Where the spray is too dense for these techniques, or the droplets

4.1 Application of Lean Flames in Internal Combustion Engines 305
are not spherical, measurements by combined Mie and LIF [173] may be possible.
In very dense s prays, it may be that so-called ‘ballistic imaging’ can provide some of
the information required [174]. Although many of these techniques can, and have,
been used in engines, optical access to the spray in the engine may be too limited in
some circumstances, or it may be desirable to measure the spray in isolation from
the engine to investigate the fundamental physics of atomisation, evaporation, and
mixing – but at realistic pressures and temperatures. For these two reasons, basic
research on sprays is being performed in high-pressure, high-temperature cells, or
rapid-compression machines (e.g., [175–177]), which give the best possible optical
access to measure spray formation, evaporation, entrainment, rates of mixture for-
mation, ignition, and soot formation. Spray break-up length can be measured in such
environments by the technique described in [178].
A particularly interesting technique is the simultaneous visualisation of the fuel
in the liquid and vapour phases by use of spectral separation of the exciplex LIF
the liquid phase from the gas-phase fluorescence: Exciplex fluorescence results from
two tracers that combine in the liquid phase only to form an exci
ted comlplex).Re-
cently, fully quantitative planar measurements have been reported in evaporating
fuel sprays under conditions of pressure and temperature relevant to engines [179],
based on full characterisation of the temperature dependence of the particular exci-
plex system used.
The measurement of the mixing of fuel vapour is usually idealised by the fa-
miliar technique of using a non-fluorescing fuel, which does not fluoresce by itself,
with the addition of a fluorescing tracer (the technique called planar laser-induced
fluorescence, PLIF), which has an evaporation rate, matched as far as possible, to
that of the fuel [180]. This technique is being extended in a number of ways. Recently,
publications have begun to appear that attempt to distinguish between various frac-
tions of a gasoline fuel, e.g. [181], and quantitative, high-temporal-resolution PLIF
[182] has been developed. Measurements of the 3D distribution of vapour have been
technically possible for some time [183], although the technique will remain expen-
sive. The fuel vapour from a diesel spray is similarly measured by a non-fluorescing
fuel ‘doped’ with a fluorescent tracer: Here, the tracers have a higher boiling point
than those used for gasoline. For diesel sprays, under certain conditions, Rayleigh
scattering has also been used to measure fuel-vapour density: Experimentally, laser
flare must be kept as low as possible with this delicate technique. So-called filtered
Rayleigh scattering avoids flare because the only wavelength detected is that which
is Doppler shifted by Brownian motion, the elastically scattered light from stationary
surfaces being filtered out [184, 185].
To measure the fuel-vapour concentration, its temperature, or the equivalence
ratio, recent developments use the general dependence of the PLIF tracer signal on
both temperature and oxygen partial pressure by making two simultaneous mea-
surements: See [186] for simultaneous measurements of temperature and residual
gas concentration based on acetone laser-induced fluorescence. When toluene is
used for temperature measurements of the gas phase, this approach has the great
convenience of needing but a single excitation wavelength [187 ]. An alternative
technique for the measurement of fuel vapour is based on the relative extinction
of two wavelengths by the vapour phase and by the liquid droplets [188] although,
because this is a line-of-sight technique, it relies on an assumption of axisymmetry
306 Lean Flames in Practice
to produce a planar measurement by the so-called ‘onion–peeling’ deconvolution
algorithm. It has nevertheless produced useful insights, as previously mentioned
[93]. A different technique for the measurement of temperature is by the addition
of micrometre-sized phosphor powder to the intake air [189]. The cooling caused
by the evaporation of directly injected fuel has been measured by rotational CARS
(R-CARS), in preference to vibrational CARS, because it offers a more accurate
measurement at low temperatures [190].
The future is likely to see increasing use of several instruments applied simul-
taneously, such as PLIF for the detection of formaldehyde as a flame-front marker,
OH* for the interface between burned and unburned gases, and tracer PLIF for fuel.
An early example is [191], which measured the local fuel concentration near the
flame front and combustion, by means of tracer PLIF and OH-PLIF, in a DI engine.
Other examples include the interaction among fuel concentration, plasma discharge,
and the flame front [192, 193]. Kim et al. [154] demonstrated the PLIF of CO, which
will also lend itself to combined measurement with other techniques.
In lean-burn SI engines, measurement of the FAR close to the time of ignition is
important: The equivalence ratio is a stochastic variable, and there is need to know
its distribution, at least, which is closely related to the subsequent growth rate of
the initial flame kernel. This can be done either by measurement of the CN and CH
radicals [83], which are formed by the spark discharge, or by measurement of the
CH/OH ratio from the chemiluminescence of the subsequent flame as it grows [79].
The growth rate of the initial flame kernel and the conditions leading to misfire are
of critical importance to stratified lean-burn engines. Recent developments [194]in
high-speed imaging and automated image analysis (which permits a conditional anal-
ysis based on IMEP) permit ‘binarised’ visualisation based on seeding the reactants
with micrometre-sized droplets and illuminating the field with a planar sheet of light:
As the reaction proceeds, the droplets are burned and a ‘negative’ image of the flame
can be seen. These have led to the conclusion that random, rare misfires (which are
nevertheless important for pollutant emission levels) and poor burn cycles are due
to local flow fluctuations that fail to move the kernel downwards into the main fuel
cloud contained in the piston bowl. [100] and [195] report a series of measurements
in a spray-guided DI engine that show the effect of spray momentum on the plasma
motion, coupled with the role of local fuel concentration on ignition stability.
In the context of LTC CI engines, the occurrence of the cool flame was mea-
sured [196, 197] by excitation with frequency-tripled Nd:YAG (neodymium:yttrium
aluminum garnet) lasers and the second-stage ignition by the disappearance of
formaldehyde and its replacement with OH. The detection of the second stage of
ignition in HCCI engines was made [198] by OH* chemiluminescence to observe the
effects of thermal stratification: however, OH* chemiluminescence is a line-of sight
technique [199]. Nevertheless, it was this technique that led to the conclusion that
it is mainly thermal stratification within the bulk gases that controls the maximum
rate of pressure rise and the unexpected conclusion that the effect of thermal strati-
fication between the bulk gases and the boundary layer has a lesser role in reducing
the maximum rate.
Broadening the scope of the discussion, there are two challenges that instru-
mentation may have to meet in future. The first is that there are differences in the
behaviour and performance between optical and all-metal engines that can arise
4.1 Application of Lean Flames in Internal Combustion Engines 307
because of effects ranging from the difference in wall temperatures to the flow
pulsations through the valves that are due to pressure waves originating in the emp-
tying and filling of other cylinders. Thus some hold that, for some applications at
least, it is preferable to measure an optically accessed cylinder that replaces one of
the cylinders in a metal multicylinder engine. This is usually less convenient than
making measurements in a single-cylinder, dedicated, optically accessed engine. In
other cases, it is either necessary or adequate to measure in all-metal engines using,
for example, access through an endoscope or through modifications to spark plugs
[200]. The second challenge is that the role of cycle-to-cycle variability and misfir-
ing is gaining importance, particularly with operation near conditions of marginal
ignition and initial flame-kernel growth. This can arise as the combustion process
becomes dependent on, for example, the details of the motion and mixing of the
first or second fuel-vapour cloud in a spray-guided SI engine or on the details of
the interaction between the high-speed spray and the convective motions in the
engine. Fortunately, high-repetition-rate lasers and high-framing-rate cameras have
recently become available, and it is now possible to measure and record several con-
secutive cycles or make CA-resolved planar measurements. An excellent example is
described in [201].
4.1.4 Concluding Remarks
As early as the turn of the 19th century, the damage done to the environment as a
result of the pollution (because of the burning of coal) caused the New York Tribune
to fulminate:
One of these days when the mischief is fully done, when our once pellucid and crystalline
atmosphere is transformed into Chicago reek, and Pittsburgh smoke and London fog, men
will begin to realize what they have lost, and will hold conventions, and pass resolutions,
and enact laws, and spend great sums of money for the undoing of the mischief and the
restoration of our atmosphere to its original state. ([202], cited by [25])
By the eve of the 1970 ‘Muskie Act’ the damage to health had become the greater
concern to a National Air Pollution Control Administration (NAPCA) epidemiolo-
gist:
What we do know is that people get killed by air pollution, and I don’t see any excuse for
there being enough air pollution to kill people. Do you? ([203], cited by [25])
r
This part of the chapter described the way in which engineering design can
exploit predominantly lean combustion, not necessarily involving a deflagration
front, in IC engines not only to reduce pollution, but also – by promoting fuel-
conversion efficiency – to husband the finite resource of petroleum-derived fuels.
r
The improvement in fuel economy consequent on lean-burn combustion, based
on the thermodynamics of fuel–air cycle analysis, is well known, as is the reduc-
tion in NO
x
emission that is due to the concomitant lower flame temperature.
r
In the 1970s and part of the 1980s, lean-burn SI combustion in throttled IC
engines was indeed able to deliver what were, at the time, great advances in fuel
economy and reduction in emissions.
308 Lean Flames in Practice
r
However, the technical interest in lean-burn combustion is framed by increas-
ingly demanding legislative constraints as well as by commercial requirements.
European legislative constraints set a fuel-economy target of 95-g CO
2
/km from
2020 and, in particular, particulate emissions that are now so low as to render
their measurement difficult. Even so, it is estimated that emissions will cause
many adverse health-related effects, including death. Lean-burn combustion
has a r ole to play in achieving these constraints – as do other technologies and
approaches outside the scope of this book.
r
For spark ignition IC engines, lean-burn combustion has been allied with
throttle-less direct injection technology to improve greatly part-load fuel econ-
omy relative to a throttled PFI stoichiometric burn equivalent. The important
virtues of the latest designs, albeit produced in limited numbers of premium
vehicles, are the achievement of
r
lean-burn combustion over a wide range of loads and speeds, and
r
low, but nevertheless close to legislative limits, emission of particulate matter.
These desirable attributes are achieved through the use of ‘spray-guided’ de-
signs exploiting the advantages of high-pressure common-rail piezoinjection.
The engine-out NO
x
emissions are low because of the use of lean burn, but
still need substantial after-treatment. Unfortunately, both of these technologies
are expensive, currently at least. However, much is becoming known, and it is
possible to be optimistic that the costs and emission levels associated with LBDI
SI engines will benefit from current research and development.
r
In the context of diesel-fuelled CI engines, the development of low temperature
combustion (LTC) holds the promise of simultaneous, large reductions in the
emissions of particulate matter and NO
x
. This is achieved through the lean
combustion of some, if not all, of the diesel fuel, together with copious amounts
of EGR. Once again, high-pressure common-rail fuel injection equipment has
played an important role in the development of this approach. Much remains to
be done, but recently conceptual models of the process have begun to emerge,
and these will undoubtedly help further research and development of LTC.
Equally exciting is the suggestion of using gasoline, or a fuel with evaporation
and octane properties similar to those of gasoline, in the context of CI engines.
This promises even further reduction in PM emissions.
r
HCCI engines hold the promise of yet greater reduction in NO
x
and particu-
late matter than LTC, possibly to the extent that neither pollutant would need
after-treatment, while maintaining high fuel economy. This would be in the form
of very lean equivalence ratios. The advantages and desirability of such com-
bustion need no further elaboration. Nevertheless, the challenges in achieving
sufficiently high IMEP while avoiding excessive rates of pressure rise remain,
with research in this field making progress as described in the main body of the
section.
r
The role of experiments – particularly using optical instrumentation – in con-
tributing to the development of lean-burn combustion in IC engines has been,
and will continue to be, substantial.
r
It has not been possible in a section of this length to discuss and illustrate
the extensive research with, and use of, computational fluid dynamics, other
than to show – very briefly and somewhat tangentially – its application in the
4.2 Application of Lean Flames in Aero Gas Turbines 309
design and development process. At a fundamental level, there will continue
to be interaction between experiments and the development of more accurate
computational models of the processes associated with DI engines (namely
fuel injection, spray atomization, droplet evaporation, charge cooling, mixture
preparation, and the control of in-cylinder motion). This will undoubtedly prove
increasingly beneficial in the future in both basic and applied research and in
commercial development.
With reference to the first quotation in this final section, made over a century
ago, humans did begin to realise what had been lost by the mid-20th century and
did indeed start to pass resolutions and enact laws – if not to restore, then at least to
mitigate some of the ‘mischief’. Although ‘great sums of money’ have been spent,
it is claimed that even greater sums of money have been saved by the introduction
of clean air legislation (see, for example, [25]). Lean-burn (SI) combustion made
a contribution to the restoration of our atmosphere, as well as to fuel economy, in
the 1970s and has started to make a contribution once more. Lean-burn combustion
is showing further promise for the medium term, in the guise of LTC, and for the
long term, as HCCI. The extent to which lean-burn combustion will play a part
in improving fuel economy and in reducing pollution, as opposed to competing
technologies, remains to be seen. But it is certain that its evaluation will come about
only through continued, and concerted, research and development.
Acknowledgements
We gratefully acknowledge the assistance of colleagues in the preparation of this
contribution: Pavlos Aleiferis, Giles Bruneaux, Alexandros Charalambides, John Dec,
Ingemar Denbratt, Mike Drake, Todd Fansler, Neil Fraser, Doug Greenhalgh, Yannis
Hardalupas, Julian Kashdan, Tim Lake, Paul Miles, Richard Pearson, Khizer Tufail,
Graham Wigley, Ernst Winklhofer, and Hua Zhao.
4.2 Application of Lean Flames in Aero Gas Turbines
By B. Jones
The combustion technology required for an aircraft engine depends on its size. The
flight length for which an engine is to be used, the proportion of operating cost
accounted for by fuel in different sectors of the engine market, and high pressure
(HP) rotor-blade size drive the trend to increase pressure ratio with thrust.
Engines can, for convenience, be grouped in thrust ranges, as shown in
Table 4.4. Within a thrust category there will always be a range of pressure ratios
because manufacturers produce families based on one engine platform. An engine
may be developed or adapted to cover a thrust range exceeding 1.5:1. Associated
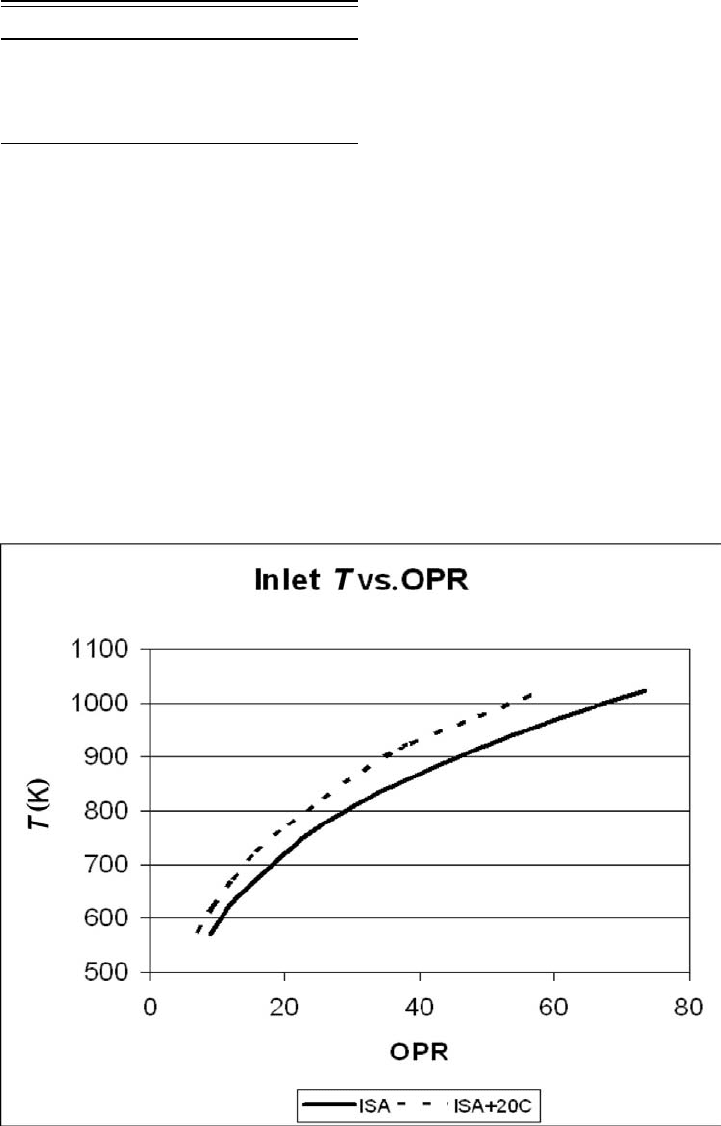
with pressure ratio is the combustor inlet temperature (see Fig. 4.50), and both these
parameters strongly affect the combustor design.
CYCLE TEMPERATURE LIMITS. In Fig. 4.50, polytropic efficiency is assumed to increase
from 0.89 at 10 overall pressure ratio (OPR) to 0.91 above 40 OPR. Compressor-disk
material will limit the maximum permissible combustor inlet temperature to 1 000 K
310 Lean Flames in Practice
Table 4.4. Engine thrust categories
Thrust (kn) OPR–a.d. 2000 OPR–a.d. 2000
180–360+ 35–43 45–55
90–180 25–35 40
45–90 20–30 35
< 45 15 20
OPR, overall pressure ratio.
for the foreseeable future. NO
x
emission will limit the turbine entry temperature
(TET) to about 1850 K in civil aircraft. The TET includes the cooling effect of the
HP turbine nozzle guide vane (NGV) cooling air admitted before the gas enters the
HP turbine. The combustor mean outlet temperature is at least 100 K higher. This
higher temperature is usually referred to as the combustor outlet temperature, which
is not uniform. The HP turbine requires a radial gas temperature profile matched to
blade stress and to avoid overheating the shrouds, which are hard t o cool, and there
will be both regular and random circumferential variations in temperature. For a
TET of 1850 K there will be hot streaks in the gas entering the nozzle guide vane at
temperatures above 2050 K arising from these non-uniformities.
Figure 4.50. Variation of combustor inlet temperature with OPR. ISA: International standard
atmosphere.
4.2 Application of Lean Flames in Aero Gas Turbines 311
DIFFERENCES BETWEEN GROUND-BASED AND AVIATION ENGINES. Ground-based en-
gines can be divided into two subdivisions – heavyweight engines designed specifi-
cally for power generation or gas pumping, and aeroderivatives, which naturally have
more in common with their progenitors. Many ground-based engines burn natural
gas – a relatively easy fuel to deal with. Diesel oil is much more difficult.
Ground-based gas turbine engines are not required to respond rapidly to a
demand for power. An aeroengine must be able to accelerate from 15% to 95%
thrust in 5s and is subject to numerous load changes during each flight. Its combustion
system is susceptible to ingestion of large amounts of water when flying through
heavy rain, and it must be capable of restarting the engine at high altitudes.
In aircraft, engine operability is a safety issue. In circumstances in which all
engines are subject to abnormal conditions, such as during landing in a tropical
storm, the passengers’ lives depend on absolute reliability of the combustor. In
ground-based installations, operability is an economic issue. These differences affect
the achievement of very low pollution.
HEAVYWEIGHT ENGINES. These often form part of a co-generation or combined cycle
plant in which exhaust heat is used for district heating or to produce steam. The cost of
increasing pressure ratio, which brings with it complex cooling systems and expensive
materials and manufacturing methods, does not justify the benefit of improved gas
generator cycle efficiency. Energy lost in the gas generator is recovered downstream.
Such engines usually operate at about 15:1 OPR and the combustors are subject to
modest inlet temperature. Size and weight do not constrain combustor design, so the
combustion system can be optimised for emissions control. Heavyweight engines
operate at constant speed to maintain generator output frequency, and the air mass
flow through the engine is restricted at low power by throttling the engine inlet.
Over most of the power range they operate at constant HP TET and the range of
the combustor FAR is relatively narrow.
AERODERIVATIVE ENGINES. These engines are based on the gas generators of large,
high-bypass-ratio engines. The fan being removed, they lose the fan precompression
of the gas generator flow, and the TET is reduced to achieve the long life required.
They serve as gas generators to drive a separate power turbine. The pressure ratio
is usually in the range 20 to 35. They are favoured in applications in which their low
weight (oil-drilling platforms, high-speed ships, power generation or gas pumping in
remote regions) or rapid starting for peak looping is of sufficient advantage to offset
their higher cost.
All ground-based gas turbines are subject to more stringent emissions regulation
than aeroengines. Regulations vary between countries – and even between states.
California and Florida have extremely stringent regulations. Regulations governing
aircraft are universal. Sometimes local variances can be met by employment of water
or steam injection in ground-based systems, but in California there are restrictions
on the use of water! Water injection has been considered during take-off in aircraft
applications (see Subsection 4.2.8).
The differences just summarised result in combustor designs for ground-based
engines that bear no resemblance to those employed in aeroengines. It is not that one
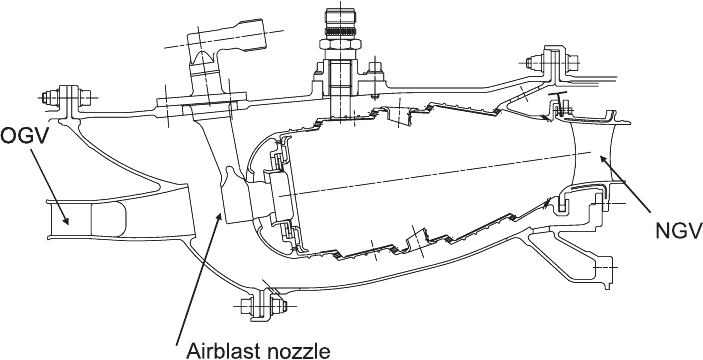
312 Lean Flames in Practice
Figure 4.51. A typical modern aero gas turbine combustor. OGV, compressor outlet guide
vane. (Courtesy of Rolls–Royce Deutschland.)
group of designers has gained a great lead over the other. The duty of the combustor
is totally different.
4.2.1 Background to the Design of Current Aero Gas Turbine Combustors
The technology on which modern aero gas turbine combustors is based was devel-
oped during the late 1970s in response to the introduction of emissions regulation and
entered service from 1985. Although the major manufacturers began in 1972 from
different starting points, determined by their past experience and the constraints
imposed by their chosen engine configurations, designs converged. Over a period of
30 years, airflow through the airblast fuel injectors has doubled, but the injector
design concepts have not changed, inlet diffusers have become much more compact,
and the materials in use today are similar to those used 30 years ago. Thermal barrier
coating improved during the 1980s to the point at which it could be depended on
not to spall. During the 1990s, significant improvements in wall-cooling technology
were introduced, notably tiled combustor walls and shallow-angled effusion-cooled
cast walls, made possible by low-cost laser drilling. The saving in cooling air al-
lowed greater control of stoichiometry within the reaction zones. Figure 4.51 is a
representative design optimised for an engine of moderate size, pressure ratio, and
cost.
Emissions reduction was achieved by incremental development, not by the in-
troduction of new concepts. Extensive research carried out since 1972, reviewed in
Subsection 4.2.6, is only now yielding alternative airworthy concepts that should en-
ter service from 2010. It is sobering that, over the past 30 years, although combustion
inefficiency at low power was reduced by an order of magnitude, the 50% reduc-
tion in NO
x
achieved at fixed engine conditions was largely eroded by the increase
in pressure ratio of 50%, which occurred over the same period. Clearly continued
incremental development is not going to eradicate NO
x
.
