Радецкий А.М., Горшкова В.П. Решение контрольных и самостоятельных работ по химии за 9 класс
Подождите немного. Документ загружается.

41
<ZjbZgl
<h^gu_jZkl\hjukbebdZlh\gZljbyb dZe bybf_xls_ehqgmx
kj_^mldwlbkhebh[jZah\Zgukbevgufhkgh\Zgb_fbkeZ[hc
dbkehlhcb\\h^_]b^jhebamxlky
2. NaOH
Z
→
Na
2
SiO
3
[
→
H
2
SiO
3
\
→
SiO
2
Z1D2+6L2
2
→
Na
2
SiO
3
+H
2
O
[1D
2
SiO
3
+2HCl
→
2NaCl+H
2
SiO
3
↓
\+
2
SiO
3
→
H
2
O+SiO
2
3. SiO
2
+C
→
CO
2
+Si
¹
(SiO
2
)=100%-10%=90%
m(SiO
2
)=0,9
⋅
]
36
]
2
60
]
SiO
+C
→
CO
2
+
o]
28
]
Si
36
o
60 28
=
o
⋅
]
Hl\_l
m(
6L ]
<ZjbZgl
Dj_fgbc\ kh_^bg_gbyoijhy\ey_lkl_i_gvhdbke_gby±
Si
-4
H
+
, Si
+4
O
−
, Si
+2
O
-2
2. Si
Z
→
Mg
2
Si
[
+HCl
→
SiH
4
\
→
SiO
2
Z6L0J
t
→
R
Mg
2
Si
[0J
2
Si+4HCl
→
2MgCl
2
+SiH
4
\6L+
4
+2O
2
→
SiO
2
+2H
2
O
3. 2NaOH+SiO
2
0
t
→
Na
2
SiO
3
+H
2
O
¹
(SiO
2
)=100%-10%=90%
m(SiO
2
)=50
⋅
]
2NaOH+
45
]
2
60
]
SiO
t
→
R
o]
23
122
]
Na SiO
+H
2
O
45
o
60 122
=
o
⋅
]
Hl\_l
m(Na
2
SiO
3
]
<ZjbZgl
Hdkb^dj_fgby6L2
2
hlghkyld dbkehlgufhdkb^Zfih_]h
k\hckl\Zf
SiO
2
+2NaOH
→
Na
2
SiO
3
+H
2
O
SiO
2
+CaO
→
CaSiO
3

42
2. Si
Z
→
SiO
2
[
→
K
2
SiO
3
\
→
H
2
SiO
3
Z6L2
2
→
SiO
2
[6L2
2
+2KOH
→
K
2
SiO
3
+H
2
O
\.
2
SiO
3
+2HCl
→
2KCl+H
2
SiO
3
↓
3. 2Mg+Si
→
Mg
2
Si
¹
(Si)=100%-5%=95%
m(Si)=0,95
⋅
]
2Mg+
57
]
28
]
Si
→
o
]
2
76
]
Mg Si
57
o
28 76
=
o
⋅
]
Hl\_l
m(Mg
2
6L ]
JZ[hlZ<uqbke_gb_fZkkubebh[t_fZijh^mdlZj_Zdpbb
ihba\_klghcfZkk_bebihh[t_f mbkoh^gh]h\_s_kl\Z
kh^_j`Zs_]hijbf_kb
1. CaCO
3
→ CaO + CO
2
ω(CaCO
3
) = 100% – 10% = 90%
m(CaCO
3
) = 600 ⋅ 0,9 = 540
]
540
]
3
100
]
CaCO
→
o]
56
]
CaO
+ CO
2
540
100
56
=
x
x =
540 56
100
⋅
= 302,4
]
Hl\_l
m(CaO) = 302,4
]
2. CaCO
3
→ CaO + CO
2
ω(CaCO
3
) = 100% – 20% = 80%
m(CaCO
3
) = 200 ⋅ 0,8 = 160
]
160
]
3
100
]
CaCO
→ CaO +
oe
2
22,4
e
CO
160
100
22
4
=
x
,
x =
160 22
4
100
⋅
,
= 35,84
e
Hl\_l9&2
2
) = 35,84
e
3. N
2
+ 3H
2
→ 2NH
3

43
ω(N
2
) = 100% – 5% = 95%
m(N
2
) = 50 ⋅ 0,95 = 47,5
]
47
,5
]
2
28
]
N
+ 3H
2
→
o]
3
217
]
2NH
⋅
47 5
28
2
17
,
=
⋅
x
o
47 5
2
17
28
,
⋅
⋅
]
η(NH
3
) =
ij
l_hj
m
8
m57,7
=
= 0,139
beb
Hl\_l
η(NH
3
) = 13,9%.
4. CaCO
3
0
t
→
CaO + CO
2
ω(CaCO
3
) = 100% – 6% = 94%
m(CaCO
3
) = 0,94 ⋅ 400 = 376
d]
376
d]
3
100
d]
CaCO
0
t
→
od]
56
d]
CaO
+ CO
2
376
100
56
=
x
o
56 376
100
⋅
d]
Hl\_l
m(CaO) = 210,56
d]
5. CaCO
3
→ CaO + CO
2
ω(CaCO
3
) = 100% – 8% = 92%
m(CaCO
3
) = 0,92 ⋅ 500 = 460
d]
460
d]
3
100
d]
CaCO
→
3
3
od] of
2
56
d]
22,4
f
CaO+ CO
460
100
56
=
x
o
460 56
100
⋅
d]
460
100
22
4
=
x
,
o
460 22
4
100
⋅
,
f
3
Hl\_l
m(CaO) = 257,6
d]
V(CO
2
) = 103
f
3
.
6.

44
o]
23
106
]
Na CO
+ 2HNO
3
→ 2NaNO
3
+ H
2
O +
2,24
e
2
22,4
e
CO
x
106
2
24
22
4
=
,
,
o
106 2
24
22 4
⋅
,
,
]
ω(Na
2
CO
3
) =
10 6
108
,
,
= 0,9815
beb
ω
ijbf
= 100% – 98,15% = 1,85%
Hl\_l
ω
ijbf
= 1,85%.
7. C + O
2
→ CO
2
ω(C) = 100% – 8% = 92%
m(C) = 500 ⋅ 0,92 = 460
]
460
]
12
]
C
+ O
2
→
oe
2
22,4
e
CO
460
12
22
4
=
x
,
o
460 22
4
12
⋅
,
e
Hl\_l
V(CO
2
) = 859
e
8.
o]
12
]
C
+ O
2
→
336
e
2
22,4
e
CO
x
12
336
22
4
=
,
o
12 336
22 4
⋅
,
]
ω
K
180
187 5,
beb
Hl\_l
ω
K
9. CaCO
3
→ CaO + CO
2
ω(CaCO
3
) = 100% – 5% = 95%
m(CaCO
3
) = 0,95 ⋅ 600 = 570
]
n(CaCO
3
) = n(CO
2
) =
570
100
= 5,7
fhev
V(CO
2
) = n ⋅ V
m
= 5,7 ⋅ 22,4 = 127,7
e
Hl\_l
n(CO
2
) = 5,7
fhev
V(CO
2
) = 127,7
e

45
10.
Ca(OH)
2
+
oe
2
22,4
e
CO
→
3
d]
3
100
d]
CaCO
+ H
2
O
x
22 4
3
100
,
=
o
3 22
4
100
⋅
,
e
ω
KH
2
) =
0 672
2000
,
beb
Hl\_l
ω
KH
2
) = 0,0336%.
11. BaCO
3
0
t
→
BaO + CO
2
ω(BaCO
3
) = 100% – 3% = 97%
m(BaCO
3
) = 0,97 ⋅ 80 = 77,6
]
BaCO
3
0
t
→
BaO + CO
2
n(BaCO
3
) = n(BaO) =
77 6
197
,
= 0,394
fhev
m(BaO) = n ⋅ M(BaO) = 0,394 ⋅ 153 = 60,3
]
Hl\_l
n(BaO) = 0,394
fhev
m(BaO) = 60,3
]
12. CaCO
3
+ 2HCl → CaCl
2
+ H
2
O + CO
2
m(CaCO
3
) = 0,95 ⋅ 60 = 57
]
n(CaCO
3
) = n(CO
2
) =
57
100
= 0,57
fhev
V(CO
2
) = n ⋅ V
m
= 0,57 ⋅ 22,4 = 12,8
e
Hl\_l
n(CO
2
) = 0,57
fhev
V(CO
2
) = 12,8
e
13.
od]
3
100
d]
CaCO
→ CaO +
22
e
2
44
e
CO
x
100
22
44
=
o
22 100
44
⋅
]
ω(CaCO
3
) =
50
54
= 0,926
beb
Hl\_l
ω(CaCO
3
) = 92,6%.
14. SiO
2
+ C → CO
2
+ Si
ω(SiO
2
) = 100% – 5% = 95%
m(SiO
2
) = 60 ⋅ 0,95 = 57
]
46
57
]
2
60
]
SiO
+ C → CO
2
+
o]
28
]
Si
57
60
28
=
x
o
57 28
60
⋅
]
Hl\_l
m(Si) = 26,6
]
JZ[hlZBlh]h\Zyihl_f_
IV
<ZjbZgl
1.
ijbagZdbkjZ\g_gby
CO
2
SiO
2
ZKhklZ\kljh_gb_ H K H
DjbklZeebq_kd Zyj_r_ldZ
fhe_dmeyjgZy
O=Si=O
DjbklZeebq_kd Zy
j_r_ldZZlhfgZy
kms_kl\m_l\\b^_
djbklZeeh\
[Nbabq_kdb_
k\hckl\Zgm
=Za[_ap\_lZ[_aaZiZoZ L\_j^h_\_s_kl\h
l_fgh]hp\_lZ
t
ie
= 1713
K
\Obfbq_kdb_
k\hckl\Z
K
O
2
+2NaOH→Na
2
CO
3
+ H
2
O
CO
2
+ CaO → CaCO
3
CO
2
+ H
2
O → H
2
CO
3
SiO
2
+ 2NaOH →
→ Na
2
SiO
3
+ H
2
O
SiO
2
+ CaO →CaSiO
3
SiO
2
+ 4HF → SiF
4
+
+ 2H
2
O
>eyhqbkldbKHhlijbf_k_cKH
2
fh`ghbkihevah\Zlv
Ca(OH)
2
—
ba\_kldh\mx\h^mb
NaOH —
]b^jhdkb^gZljby
Ca(OH)
2
+ 2CO
2
→
Ca(HCO
3
)
2
Ca(OH)
2
+ CO
2
→
CaCO
3
+ H
2
O
NaOH + CO
2
→
NaHCO
3
2NaOH + CO
2
→
Na
2
CO
3
+ H
2
O
3. Na
2
CO
3
1)
→
CaCO
3
2)
→
CO
2
3)
→
Ca(HCO
3
)
2
4)
→
CO
2
1) Na
2
CO
3
+ Ca(OH)
2
→
CaCO
3
+ 2NaOH
2) CaCO
3
0
t
→
CaO + CO
2
3) 2CO
2
+ Ca(OH)
2
→
Ca(HCO
3
)
2
4) Ca(HCO
3
)
2
→
CaCO
3
+ CO
2
+ H
2
O
4. CaCO
3
0
t
→
CaO + CO
2
ω
(CaCO
3
) = 100% – 6% = 94%
m(CaCO
3
) = 400
⋅
0,94 = 376
]
47
37
6
d
]
3
10
0
d
]
CaCO
0
t
→
CaO +
o
e
2
22,4
e
CO
376
100
22
4
=
x
,
o
376 22
4
100
⋅
,
e
Hl\_l
V(CO
2
) = 84,2
e
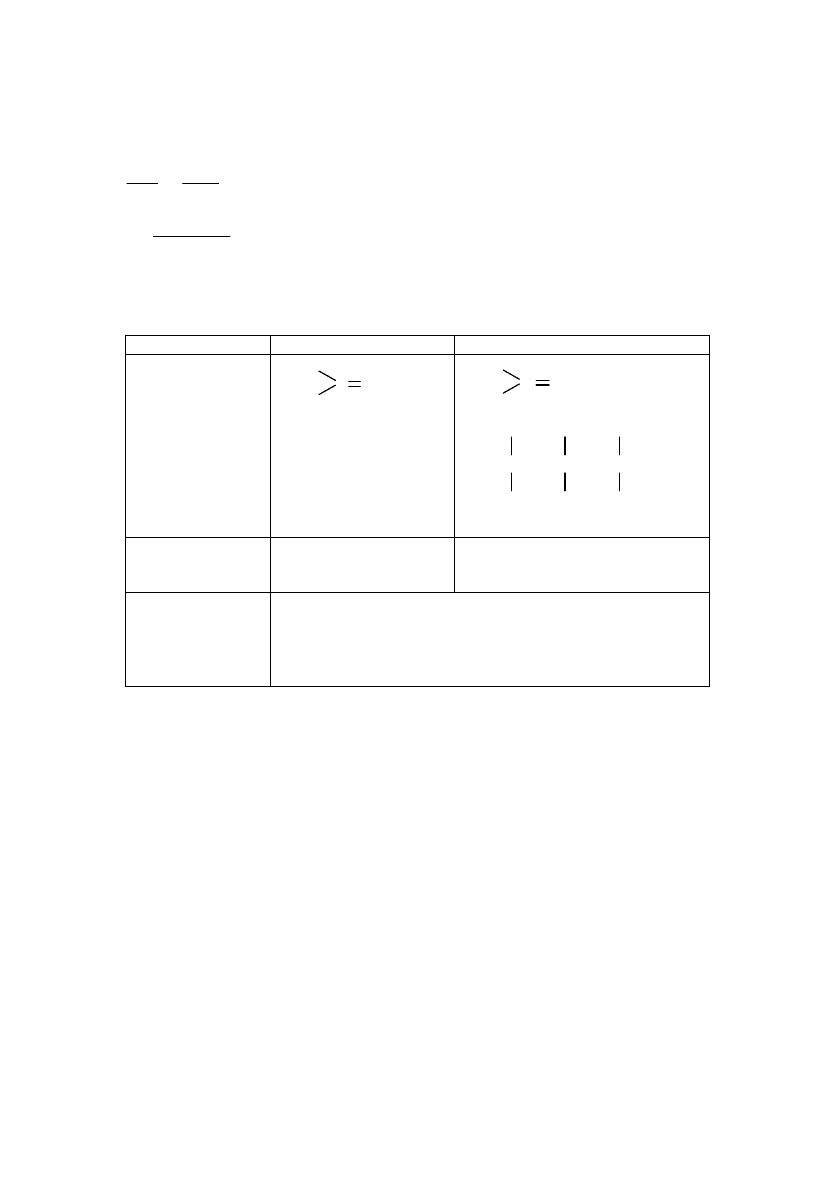
<ZjbZgl
1.
ijbagZdbkjZ\g_gby
H
2
CO
3
H
2
SiO
3
ZKhklZ\kljh_gb_
+
²
2
+
²
2
&
2
+
²
2
+
²
2
6
L
2
²
2
²
6
L
²
2
²
6
L
²
2
²
6
L
²
2
²
2
+
²
2
+
²
2
+
²
2
+
²
2
+
²
2
+
²
y\ey_lkyihebf_jhf
[Nbabq_kdb_
k\hckl\Z
Kms_kl\m_llhevdh\
jZkl\hj_[_kp\_lguc
jZkl\hj[_aaZiZoZ
Kms_kl\m_l\\b^_ihebf_jguo
p_i_cdheehb^gucjZkl\hj
\Obfbq_kdb_
k\hckl\Z
H
2
SiO
3
+ 2NaOH
→
Na
2
SiO
3
+ 2H
2
O
H
2
CO
3
+ 2NaOH
→
Na
2
CO
3
+ 2H
2
O
H
2
SiO
3
0
t
→
H
2
O + SiO
2
H
2
CO
3
0
t
→
H
2
O + CO
2
2. Ca(NO
3
)
2
+ K
2
CO
3
→ CaCO
3
↓ + 2KNO
3
Ca(NO
3
)
2
+ MgSO
4
→ CaSO
4
↓ + Mg(NO
3
)
2
HkZ^hdij_^klZ\ey_lkh[hckf_kv
CaCO
3
b
CaSO
4
.
3. CO
2
1)
→
Na
2
CO
3
2)
→
NaHCO
3
3)
→
CO
2
4)
→
CaCO
3
1) CO
2
+ 2NaOH → Na
2
CO
3
+ H
2
O
2) Na
2
CO
3
+ H
2
O + CO
2
→ 2NaHCO
3
3) 2NaHCO
3
0
t
→
Na
2
CO
3
+ H
2
O + CO
2
4) CO
2
+ CaO → CaCO
3
4. SiO
2
+ 2Mg → Si + 2MgO
ω(SiO
2
) = 100% – 3% = 97%
m(SiO
2
) = 30 ⋅ 0,97 = 29,1
]
29,1
]
2
60
]
SiO
+ 2Mg →
o]
28
]
Si
+ 2MgO
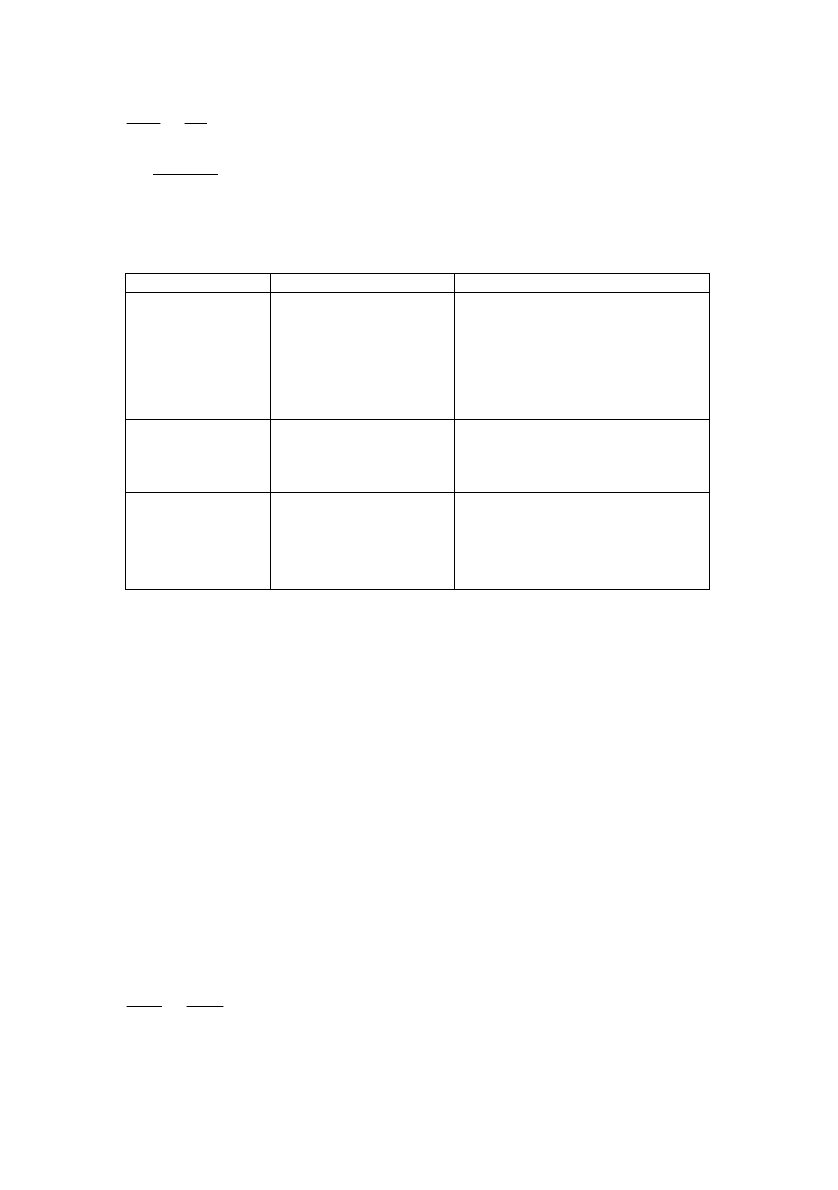
48
291
60
28
,
=
x
o
291 28
60
,
⋅
]
Hl\_l
m(Si) = 13,6
]
<ZjbZgl
1.
ijbagZdbkjZ\g_gby
=jZnbl DjbklZeebq_kd bcdj_fgbc
ZKhklZ\b
kljh_gb_
:lhfum]e_jh^Z
gZoh^ylky\ khklhygbb
sp
2
-
]b[jb^baZpbb
ZlhfZkh_^bgyxlky^jm]k
^jm]hfZ c hklZ_lky
k\h[h^gufZlhfu
jZkiheh`_gukehyfb
KlmdlmjZZgZeh]bqgZkljmdlmj_
ZefZaZZlhfgZydjbklZeebq_kdZy
j_r_ldZZlhfudj_fgby\
khklhygbb
sp
3
-
]b[jb^baZpbb
y\ey_lkyihemijh\h^gbdhf
[Nbabq_kdb_
k\hckl\Z
L\_j^h_lm]hieZ\dh_
\_s_kl\hk_jh]h p\_lZ
`bjgh_gZhsmiv
we_dljhijh\h^_g
L\_j^h_l_fghk_jh_\_s_kl\hk
f_lZeebq_kdbf[e_kdhf
t
ie
= 1420
K
\Obfbq_kdb_
k\hckl\Z
C + O
2
→
CO
2
C + 2H
2
→
CH
4
C + 2F
2
→
CF
4
2C + Ca
→
CaC
2
C + CuO
→
Cu + CO
Si + O
2
→
SiO
2
Si + 2F
2
→
SiF
4
Si + 2Mg
→
Mg
2
Si
Si+2NaOH+H
2
O
→
Na
2
SiO
3
+ 2H
2
2. Na
2
CO
3
+ BaCl
2
→
2NaCl + BaCO
3
↓
Na
2
SO
4
+ BaCl
2
→
BaSO
4
↓
+ 2NaCl
BaCO
3
+ 2HNO
3
→
Ba(NO
3
)
2
+ CO
2
↑
+ H
2
O
BaSO
4
+ 2HNO
3
→
/
3. CO
2
1)
→
CaCO
3
2)
→
Ca(HCO
3
)
2
3)
→
CaCO
3
4)
→
CO
2
1) CO
2
+ CaO
→
CaCO
3
2) CaCO
3
+ H
2
O + CO
2
→
Ca(HCO
3
)
2
3) Ca(HCO
3
)
2
0
t
→
CaCO
3
+ H
2
O + CO
2
4) CaCO
3
+ 2HCl
→
CaCl
2
+ H
2
O + CO
2
4. CaCO
3
+ 2HNO
3
→
Ca(NO
3
)
2
+ H
2
O + CO
2
ω
(CaCO
3
) = 100% – 3% = 97%
m(CaCO
3
) = 20
⋅
0,97 = 19,4
]
19,4
]
3
100
d]
CaCO
+ 2HNO
3
→
Ca(NO
3
)
2
+ H
2
O +
oe
2
22,4
e
CO
19 4
100
22
4
,
,
=
x
49
o
19 4
22
4
100
,
,
⋅
e
Hl\_l
V(CO
2
) = 4,35
e
<ZjbZgl
1.
ijbagZdbkjZ\g_gby
KH KH
2
Nbabq_kd b _
k\hckl\Z
=Za[_ap\_lZ[_aaZiZoZ
k ijbf_kyfbbf__l
oZjZdl_jgucaZiZo
y^h\ble_]q_\ha^moZ
=Za[_ap\_lZ[_aaZiZoZ
fZehjZkl\hjbf\\h^_ly`_e__
\ha^moZ
Obfbq_kdb_
k\hckl\Z
CuO+CO
→
Cu+ CO
2
2CO + O
2
→
2CO
2
CO
2
+ H
2
O
-
H
2
CO
3
CO
2
+ 2NaOH → Na
2
CO
3
+ H
2
O
CO
2
+ CaO → CaCO
3
2.
Kh^_j`bfh_dZ`^hcijh[bjdbfh`ghhij_^_eblvijbihfhsb
jZkl\hjZ
HCl.
Na
2
SiO
3
+ 2HCl
→
2NaCl + H
2
SiO
3
↓
Na
2
CO
3
+ 2HCl
→
2NaCl + H
2
O + CO
2
↑
Na
2
SO
4
+ 2HCl
→
/
3. SiO
2
1)
→
K
2
SiO
2)
→
H
2
SiO
3
3)
→
SiO
2
4)
→
Si
1) SiO
2
+ 2KOH
→
K
2
SiO
3
+ H
2
O
2) K
2
SiO
3
+ 2HCl
→
2KCl + H
2
SiO
3
↓
3) H
2
SiO
3
0
t
→
H
2
O + SiO
2
4) SiO
2
+ C
→
Si + CO
2
4. Si + O
2
→
SiO
2
ω
(Si) = 100% – 5% = 95%
m(Si) = 0,95
⋅
60 = 57
]
57
]
28
]
Si
+ O
2
→
o]
2
60
]
SiO
57
28
60
=
x
o
57 60
28
⋅
]
Hl\_l
m(SiO
2
) = 122,14
]

50
L_fZ
V
H[sb_k\hckl\Zf_lZeeh\
JZ[hlZKihkh[uihemq_gbyf_lZeeh\
<ZjbZgl
1.
+1 -2
2
hdbkebl_ev
Cu O
+
0
\hkklZgh\bl_ev
C
→2Cu
0
+ C
+
2
O
–2
+1 -2
2
hdbkebl_ev
Cu O
+
0
\hkklZgh\bl_ev
C
→ Cu
0
+ C
+2
O
–2
2. 3MnO
2
+ 4Al → 2Al
2
O
3
+ 3Mn
ω(MnO
2
) = 100% – 5% = 95%
m(MnO
2
) = 90 ⋅ 0,95 = 85,5
]
85,5
]
2
3 87
]
3MnO
⋅
+ 4Al → 2Al
2
O
3
+
o]
3 55
]
3Mn
⋅
855
3 87
3
55
,
⋅
=
⋅
x
o
855 3
55
3 87
,
⋅
⋅
⋅
]
Hl\_l
m(Mn) = 54,05
]
<ZjbZgl
1.
+3
23
hdbkebl_ev
Cr O
+
0
\hkklZgh\bl_ev
2Al
→ Al
2
+3
O
3
–2
+ 2Cr
0
2.
960
]
23
160
]
Fe O
+ 3CO →
o]
256
]
2Fe
⋅
+ 3CO
2
960
160
2
56
=
⋅
x
o
960 2
56
160
⋅
⋅
]
m
ij
= η(Fe) ⋅ m
l_hj
= 0,9 ⋅
]
Hl\_l
m(Fe) = 604,8
]
<ZjbZgl
Z
+4 -2
2
hdbkebl_ev
Pb O
+
+2 -2
\hkklZgh\bl_ev
2C O
→ Pb
0
+ 2C
+4
O
2
–2
[
+2 -2
hdbkebl_ev
Cu O
+
+2 -2
\hkklZgh\bl_ev
CO
→ Cu
0
+ C
+4
O
2
–2
2.
o]
23
152
]
Cr O
+ 2Al → Al
2
O
3
+
52
]
252
]
2Cr
⋅
