Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

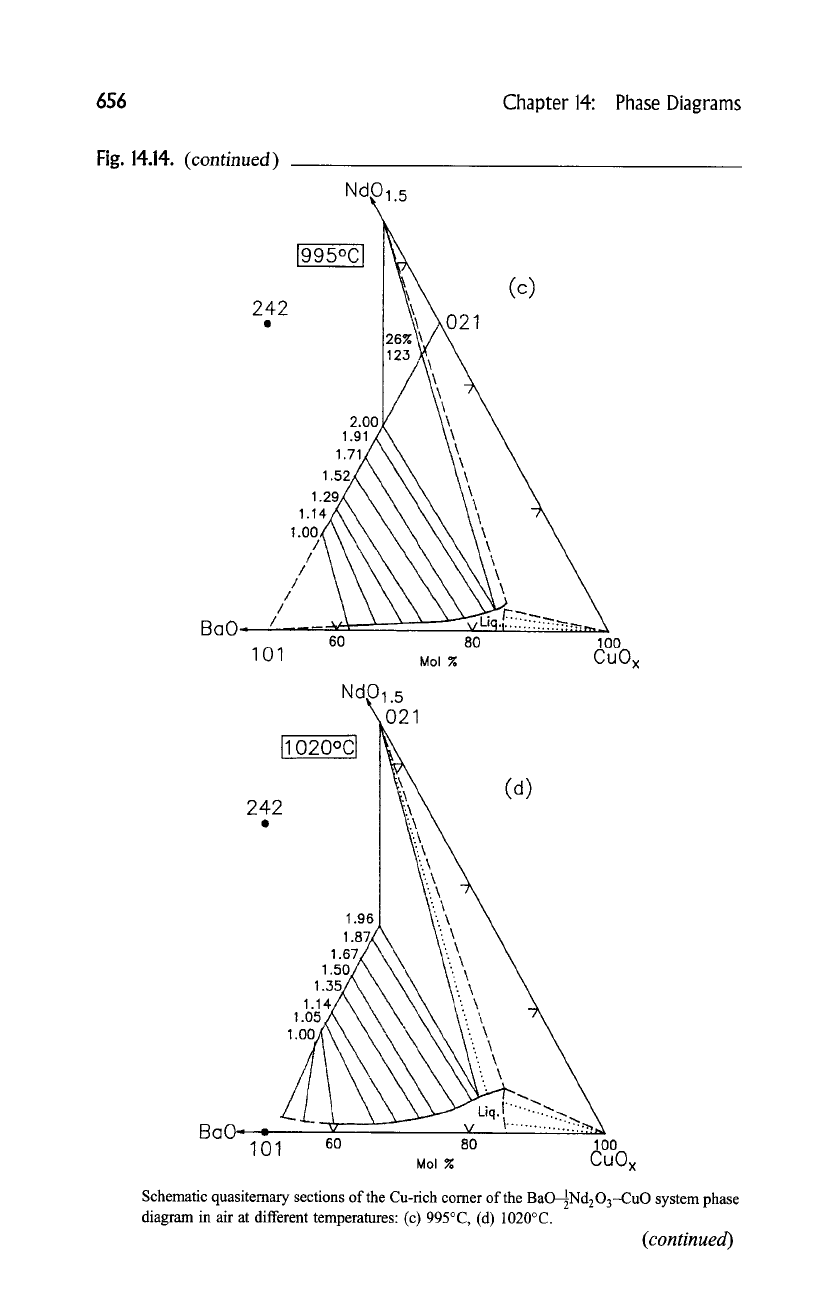
656
Chapter 14: Phase Diagrams
Fig. 14.14.
(continued)
Ndl)l.5
1995ocl
242 I (C)
2.00
171 /
1.sy(X
1.29// k k
,,'\\\\
60 80 1 O0
1 01 Mol ~. CuOx
BaO~
242
Nd~01.5
!1020ocl ~21
(d)
1.96
1.87/(
1.1
'
\\
\\
BOO-:- -- ~ v , ....... --. .....
::~,-',
I 01 60 80 100
Mol ~. CUOx
Schematic quasitemary sections of the Cu-rich comer of the BaO--~Nd203--CuO system phase
diagram in air at different temperatures: (c) 995~ (d) 1020~
(continued)
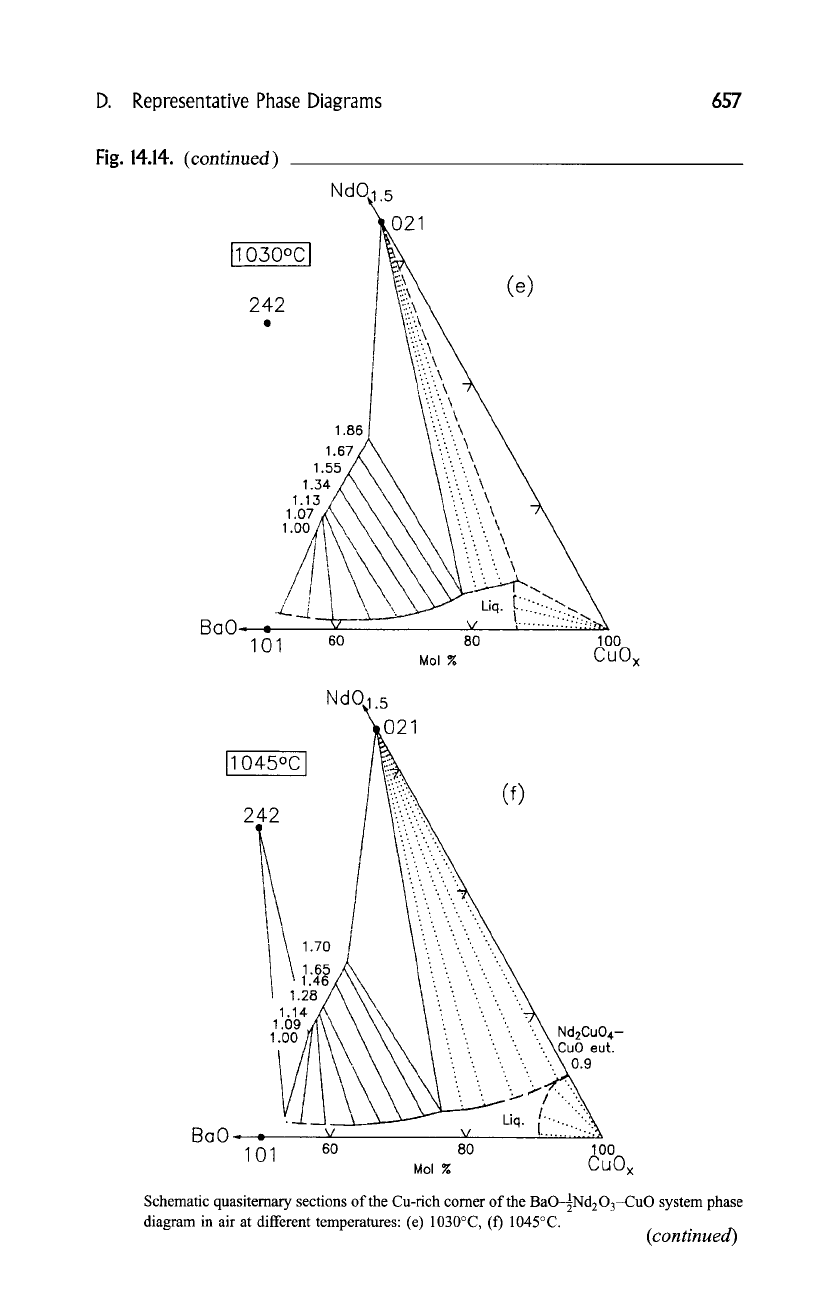
D.
Representative Phase Diagrams
657
Fig.
14.14.
(continued)
1!o3oo01
24-2
@
Nd
1.86
1.67~
1.55 X' '
1.34
/ \
1.13 /' \ '
1.07/,\ 'X
1.00/1\\ '
BaO~ =
101 60
.5
~t021
(e)
".._ ... '\\
_._ .. \
k --.. \
--.".... \\
\ ".....-._ '... \\
', __-.
\.N~" Uq [.? ....... ..
~"~- " i "'" .... "":""
V
i
.......... .::
8O
Mol %
100
CuOx
11045~
242
'!
1.14
1.09
1.00/
Nd01.5
\o,1
/
1.70 /
(f)
9 . . . 9
~ -.....
9 ,
Ba 0--- -- v v
101 60
80
Mol ~,
9 9 -j
9
"""
~9N d2C u 04-
9 9 "
eut.
Liq. 1.. "-
I...'.'.'.'.'.':
100
CuOx
Schematic quasiternary sections of the Cu-rich corner of the 1
BaO-~Nd203-CuO system phase
diagram in air at different temperatures: (e) 1030~ (f) 1045~
(continued)
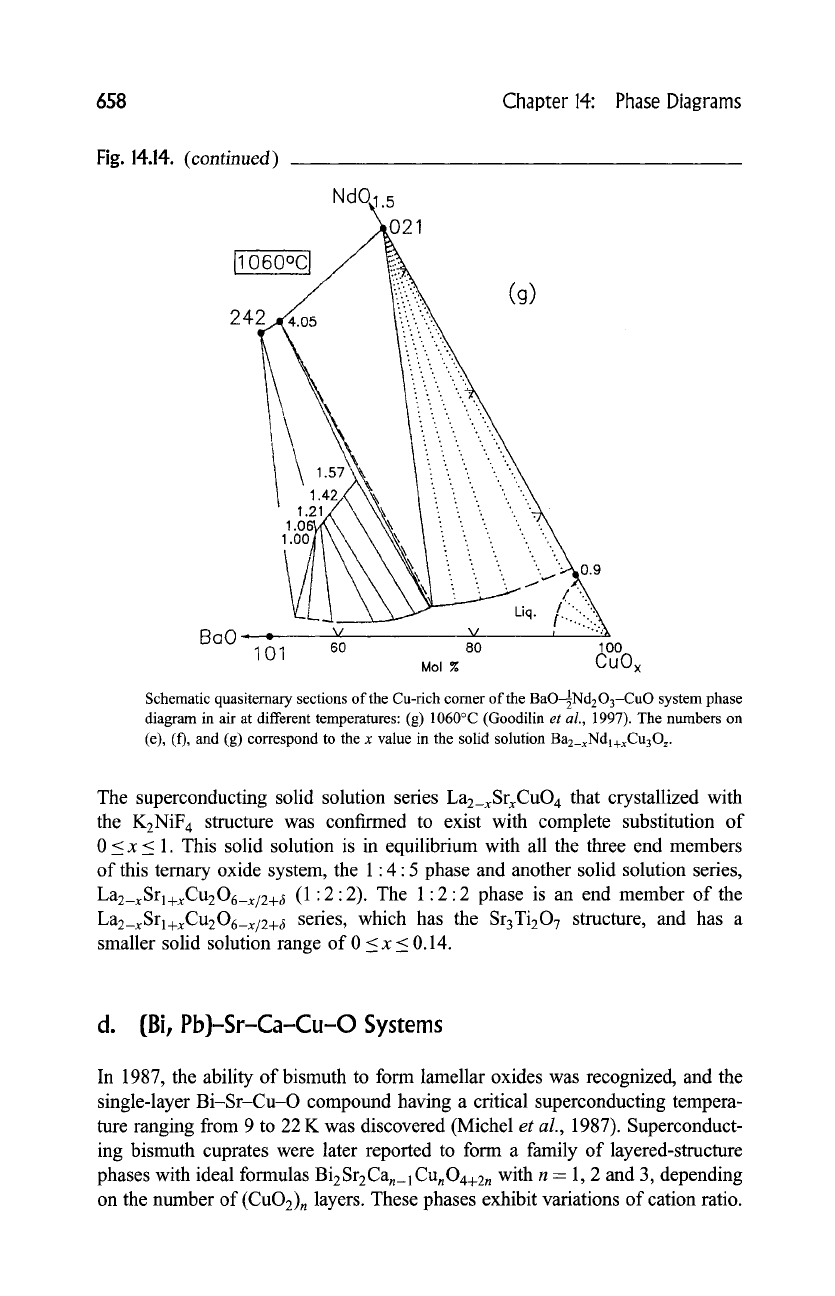
658 Chapter 14: Phase Diagrams
Fig. 14.14.
(continued)
i1060ocl
242
Nd
.5
~021
(g)
1.0{
1.00
BoO ~ = v
101 60
i : i ii iiiiii
~iq I'/"-i''" - "'- "%".
80 1 O0
Mol ~ CuOx
Schematic quasitemary sections of the Cu-rich comer of the BaOINd203-CuO system phase
diagram in air at different temperatures: (g) 1060~ (Goodilin
et al.,
1997). The numbers on
(e), (f), and (g) correspond to the x value in the solid solution Ba2_xNdl+xCu3Oz.
The superconducting solid solution series
La2_xSrxCuO 4
that crystallized with
the K2NiF 4 structure was confirmed to exist with complete substitution of
0 < x < 1. This solid solution is in equilibrium with all the three end members
of this ternary oxide system, the 1"4"5 phase and another solid solution series,
La2_xSrl+xCu206_x/2+ 6
(1"2"2). The 1 "2"2 phase is an end member of the
La2_xSr l+xCu206_x/2+
6
series, which has the
Sr 3Ti
20
7
structure, and has a
smaller solid solution range of 0 < x < 0.14.
d. {Bi, Pb}-Sr-Ca-Cu-O Systems
In 1987, the ability of bismuth to form lamellar oxides was recognized, and the
single-layer Bi-Sr-Cu-O compound having a critical superconducting tempera-
ture ranging from 9 to 22 K was discovered (Michel
et al.,
1987). Superconduct-
ing bismuth cuprates were later reported to form a family of layered-structure
phases with ideal formulas Bi2Sr2Can_ 1CunOa+2n with n = 1, 2 and 3, depending
on the number of (CuO2)n layers. These phases exhibit variations of cation ratio.
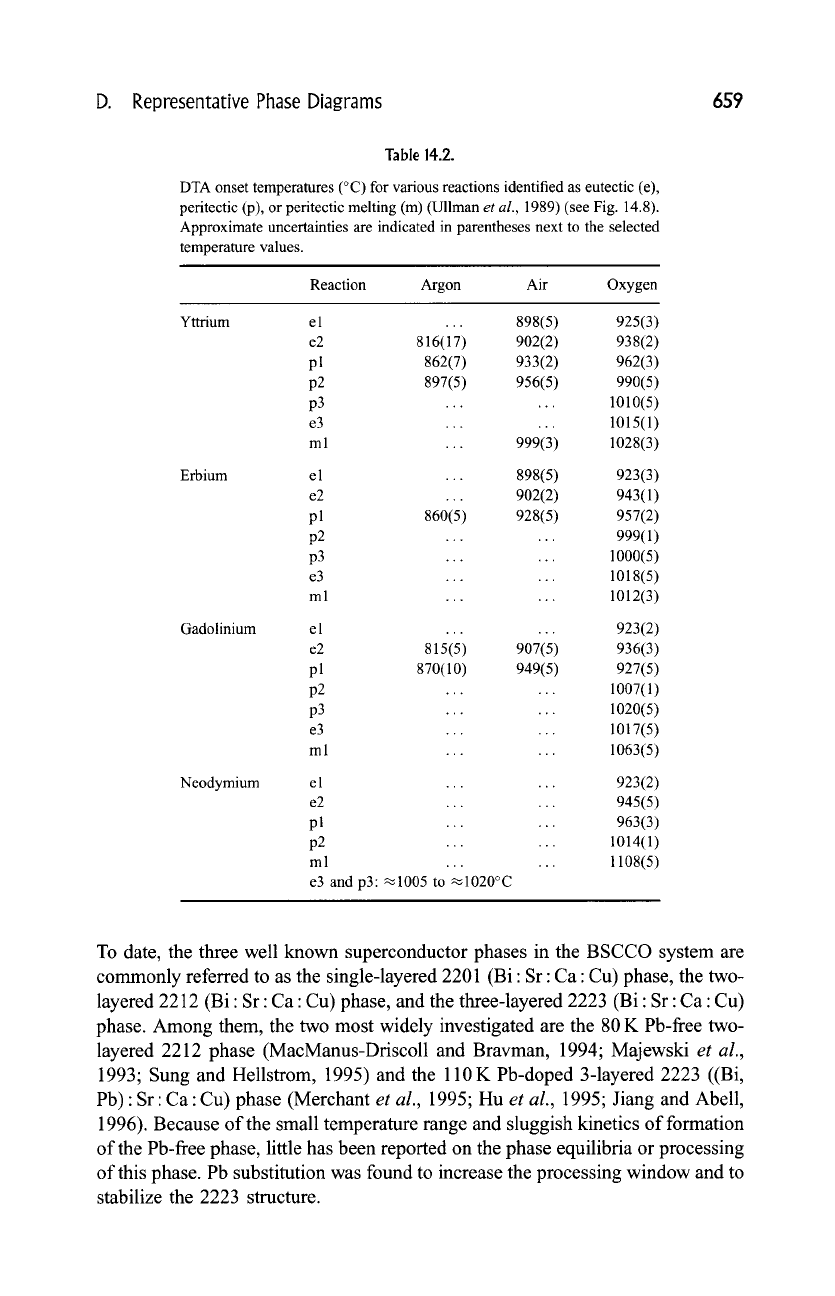
D. Representative Phase Diagrams
659
Table 14.2.
DTA onset temperatures (~ for various reactions identified as eutectic (e),
peritectic (p), or peritectic melting (m) (Ullman
et al.,
1989) (see Fig. 14.8).
Approximate uncertainties are indicated in parentheses next to the selected
temperature values.
Reaction Argon Air Oxygen
Yttrium
Erbium
Gadolinium
Neodymium
el ... 898(5) 925(3)
e2 816(17) 902(2) 938(2)
pl 862(7) 933(2) 962(3)
p2 897(5) 956(5) 990(5)
p3 ...... 1010(5)
e3 ...... 1015(1)
ml ... 999(3) 1028(3)
el ... 898(5) 923(3)
e2 ... 902(2) 943(1)
p 1 860(5) 928(5) 957(2)
p2 ...... 999(1)
p3 ...... 1000(5)
e3 ...... 1018(5)
ml ...... 1012(3)
el ...... 923(2)
e2 815(5) 907(5) 936(3)
pl 870(10) 949(5) 927(5)
p2 ...... 1007(1)
p3 ...... 1020(5)
e3 ...... 1017(5)
ml ...... 1063(5)
el ...... 923(2)
e2 ...... 945(5)
pl ...... 963(3)
p2 ...... 1014(1)
ml ...... 1108(5)
e3 and p3:~1005 to ~1020~
To date, the three well known superconductor phases in the BSCCO system are
commonly referred to as the single-layered 2201 (Bi : Sr: Ca : Cu) phase, the two-
layered 2212 (Bi : Sr: Ca : Cu) phase, and the three-layered 2223 (Bi : Sr : Ca : Cu)
phase. Among them, the two most widely investigated are the 80 K Pb-free two-
layered 2212 phase (MacManus-Driscoll and Bravman, 1994; Majewski
et al.,
1993; Sung and Hellstrom, 1995) and the 110 K Pb-doped 3-layered 2223 ((Bi,
Pb) : Sr: Ca : Cu) phase (Merchant
et al.,
1995; Hu
et al.,
1995; Jiang and Abell,
1996). Because of the small temperature range and sluggish kinetics of formation
of the Pb-free phase, little has been reported on the phase equilibria or processing
of this phase. Pb substitution was found to increase the processing window and to
stabilize the 2223 structure.
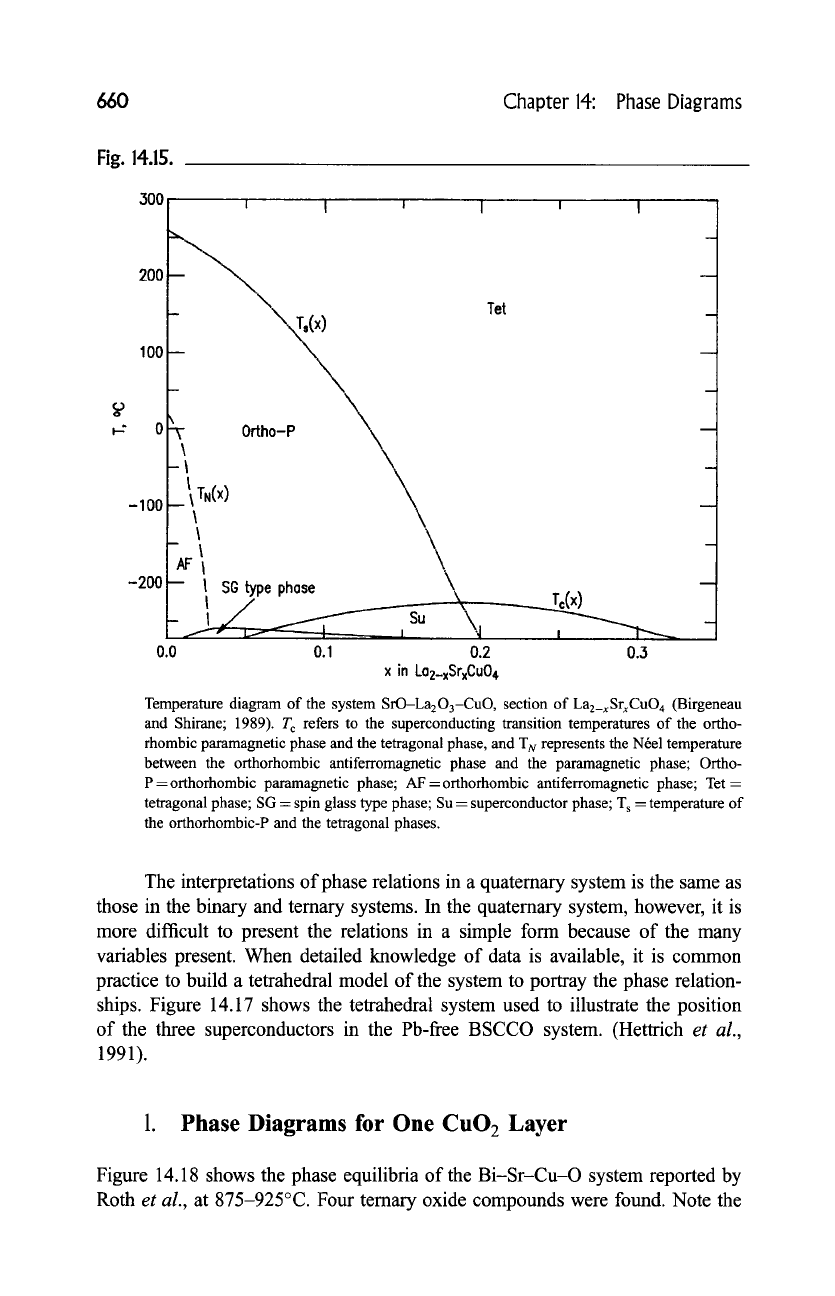
660 Chapter 14: Phase Diagrams
Fig. 14.15.
.o
-100
-200
300-
200
\
~xT,(x
100 _ -- ~~
0.0
!
I ' !
let
~- Ortho-P
_~ \
--i TN(x)
'
- ~
AF I
-- I SG
type phase
\
0.1 0.2 0.3
x in Laz-xSrxCu04
Temperature diagram of the system SrO-La203-CuO , section of La2_xSrxCuO 4 (Birgeneau
and Shirane; 1989). T c refers to the superconducting transition temperatures of the ortho-
rhombic paramagnetic phase and the tetragonal phase, and "IN represents the Nrel temperature
between the orthorhombic antiferromagnetic phase and the paramagnetic phase; Ortho-
P =orthorhombic paramagnetic phase; AF =orthorhombic antiferromagnetic phase; Tet =
tetragonal phase; SG = spin glass type phase; Su = superconductor phase; T s -- temperature of
the orthorhombic-P and the tetragonal phases.
The interpretations of phase relations in a quaternary system is the same as
those in the binary and ternary systems. In the quaternary system, however, it is
more difficult to present the relations in a simple form because of the many
variables present. When detailed knowledge of data is available, it is common
practice to build a tetrahedral model of the system to portray the phase relation-
ships. Figure 14.17 shows the tetrahedral system used to illustrate the position
of the three superconductors in the Pb-free BSCCO system. (Hettrich
et al.,
1991).
1. Phase Diagrams for One Cu02 Layer
Figure 14.18 shows the phase equilibria of the Bi-Sr-Cu-O system reported by
Roth
et al.,
at 875-925~ Four ternary oxide compounds were found. Note the
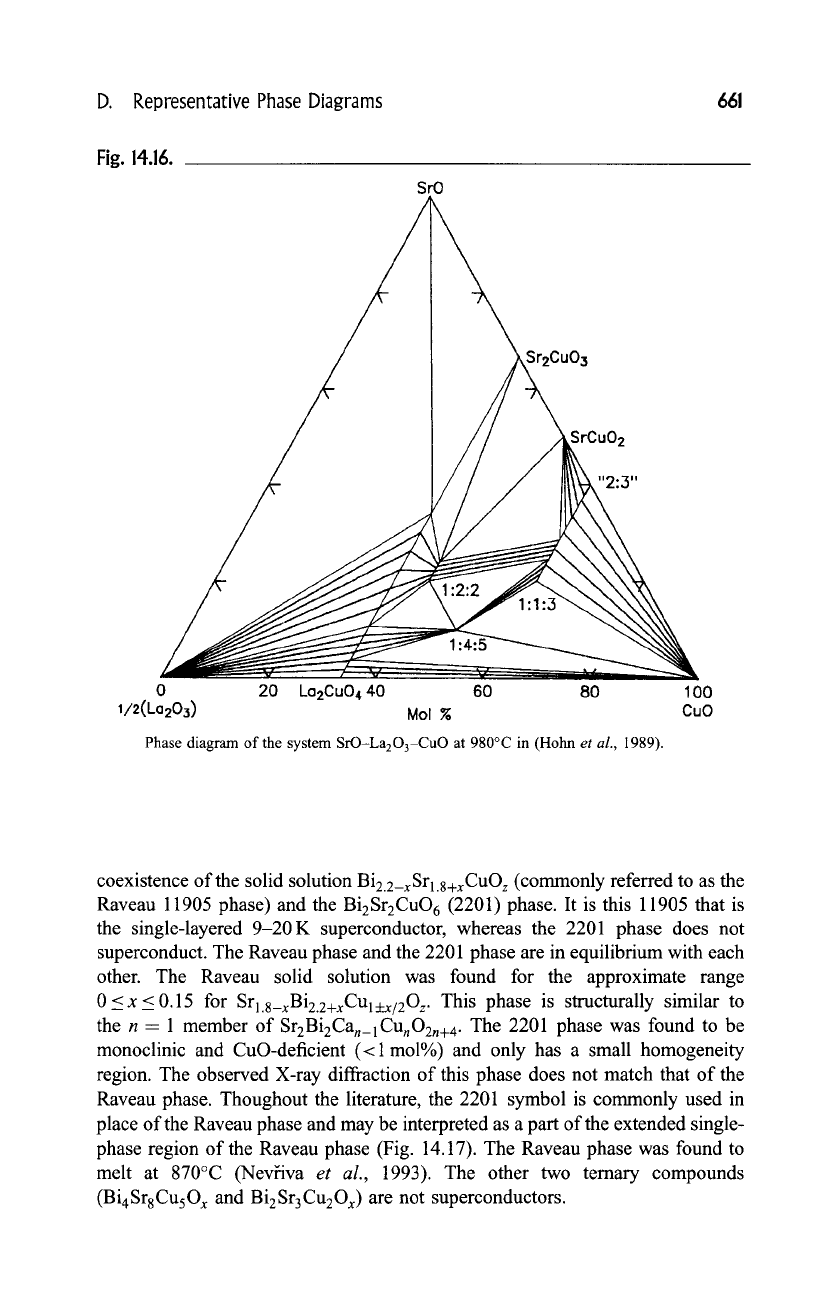
D. Representative Phase Diagrams
661
Fig. 14.16.
SrO
Sr2Cu03
SrCuO 2
"2:,3"
0 20 ka2Cu0440 60 80 I00
I/2(La203) Mol % CuO
Phase diagram of the system SrO-La203-CuO at 980~ in (Hohn
et al.,
1989).
coexistence of the solid solution
Bi2.2_xSrl.8+xCuO z
(commonly referred to as the
Raveau 11905 phase) and the Bi2Sr2CuO 6 (2201) phase. It is this 11905 that is
the single-layered 9-20 K superconductor, whereas the 2201 phase does not
superconduct. The Raveau phase and the 2201 phase are in equilibrium with each
other. The Raveau solid solution was found for the approximate range
0<x_<0.15 for
Srl.8_xBi2.2+xCUl:Lx/20 z.
This phase is structurally similar to
the n- 1 member of
SrzBizCan_]CunO2n+4.
The 2201 phase was found to be
monoclinic and CuO-deficient (<1 mol%) and only has a small homogeneity
region. The observed X-ray diffraction of this phase does not match that of the
Raveau phase. Thoughout the literature, the 2201 symbol is commonly used in
place of the Raveau phase and may be interpreted as a part of the extended single-
phase region of the Raveau phase (Fig. 14.17). The Raveau phase was found to
melt at 870~ (Nevfiva
et al.,
1993). The other two ternary compounds
(BiaSr8CusO x and Bi2Sr3Cu2Ox) are not superconductors.
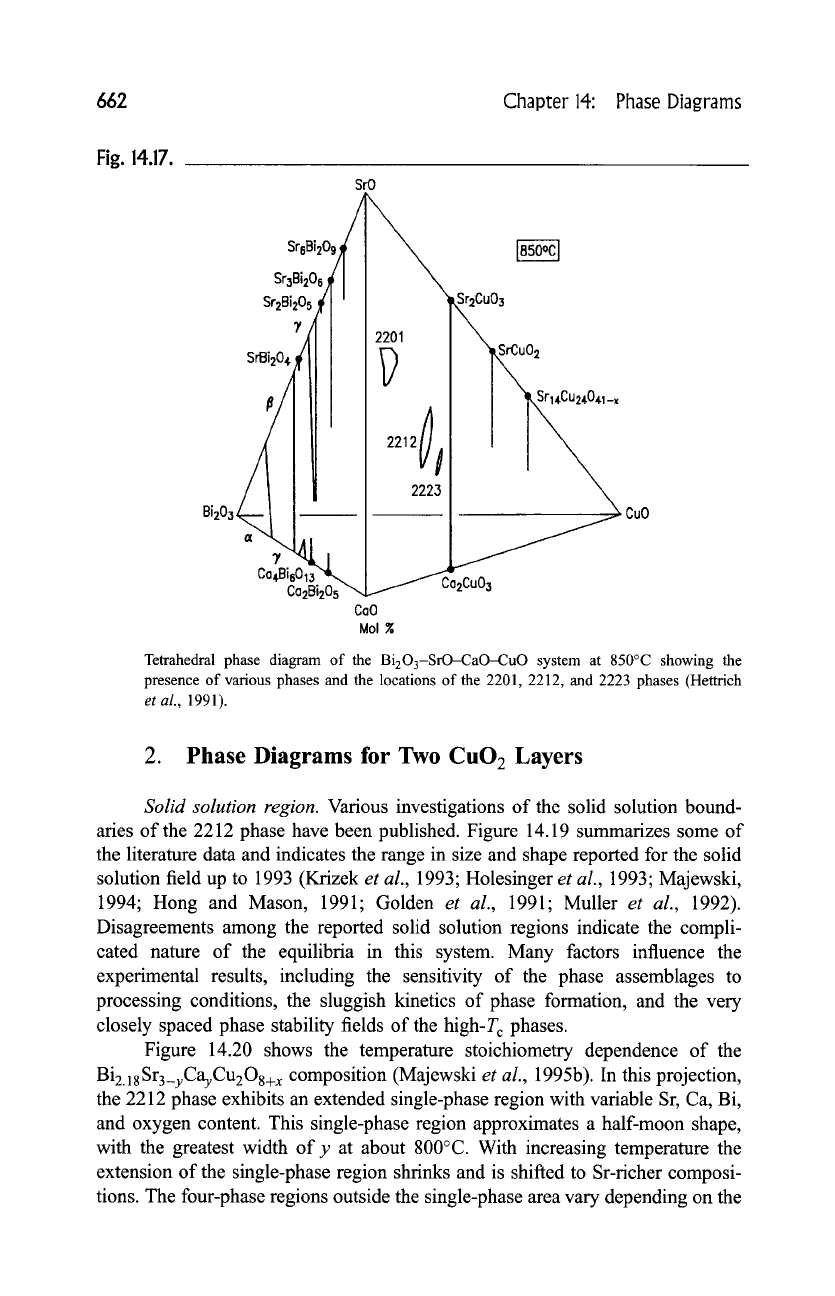
662 Chapter 14:
Phase
Diagrams
Fig. 14.17.
SrO
Sr3ai=o'~l I
jl, I
/I !i I I i
"l,,Sr~4cu=4o,,~-,
/I Ii I 2223
CoO
Mol g
CuO
Tetrahedral phase diagram of the
Bi203-SrO-CaO-CuO
system at 850~ showing the
presence of various phases and the locations of the 2201, 2212, and 2223 phases (Hettrich
et al.,
1991).
2. Phase Diagrams for Two CuO 2 Layers
Solid solution region.
Various investigations of the solid solution bound-
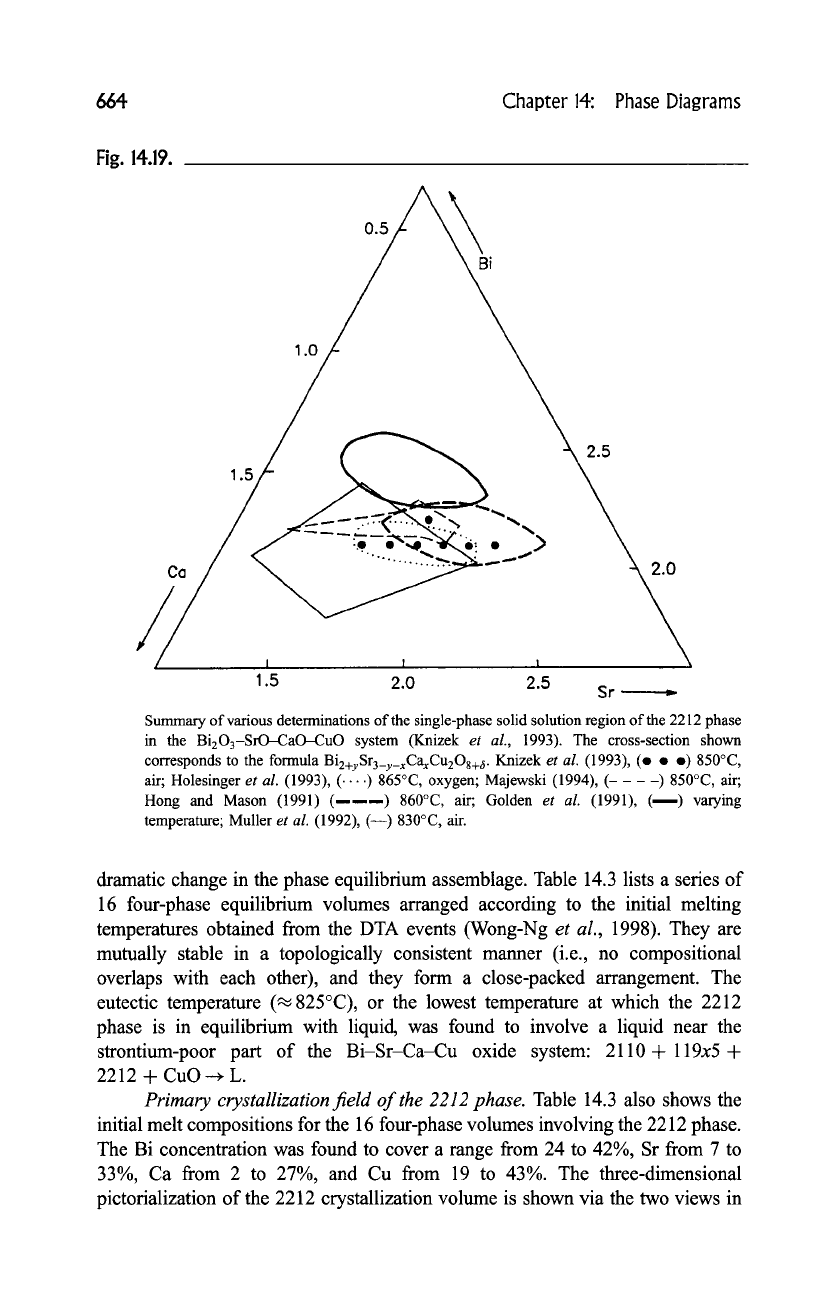
aries of the 2212 phase have been published. Figure 14.19 summarizes some of
the literature data and indicates the range in size and shape reported for the solid
solution field up to 1993 (Krizek
et al.,
1993; Holesinger
et al.,
1993; Majewski,
1994; Hong and Mason, 1991; Golden
et al.,
1991; Muller
et al.,
1992).
Disagreements among the reported solid solution regions indicate the compli-
cated nature of the equilibria in this system. Many factors influence the
experimental results, including the sensitivity of the phase assemblages to
processing conditions, the sluggish kinetics of phase formation, and the very
closely spaced phase stability fields of the high-T c phases.
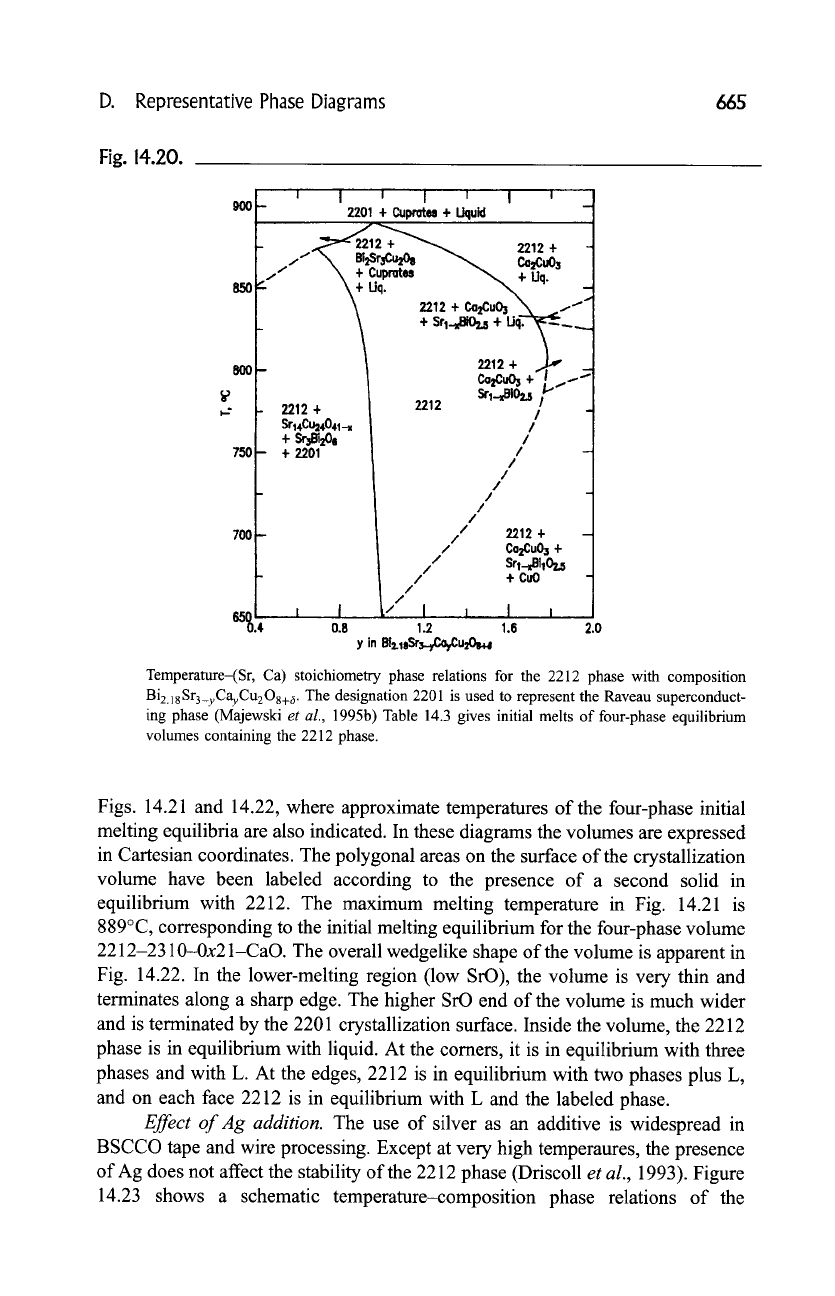
Figure 14.20 shows the temperature stoichiometry dependence of the
Bi2.18Sr3_yCayCu208+ x composition (Majewski
et al.,
1995b). In this projection,
the 2212 phase exhibits an extended single-phase region with variable Sr, Ca, Bi,
and oxygen content. This single-phase region approximates a half-moon shape,
with the greatest width of y at about 800~ With increasing temperature the
extension of the single-phase region shrinks and is shifted to Sr-richer composi-
tions. The four-phase regions outside the single-phase area vary depending on the
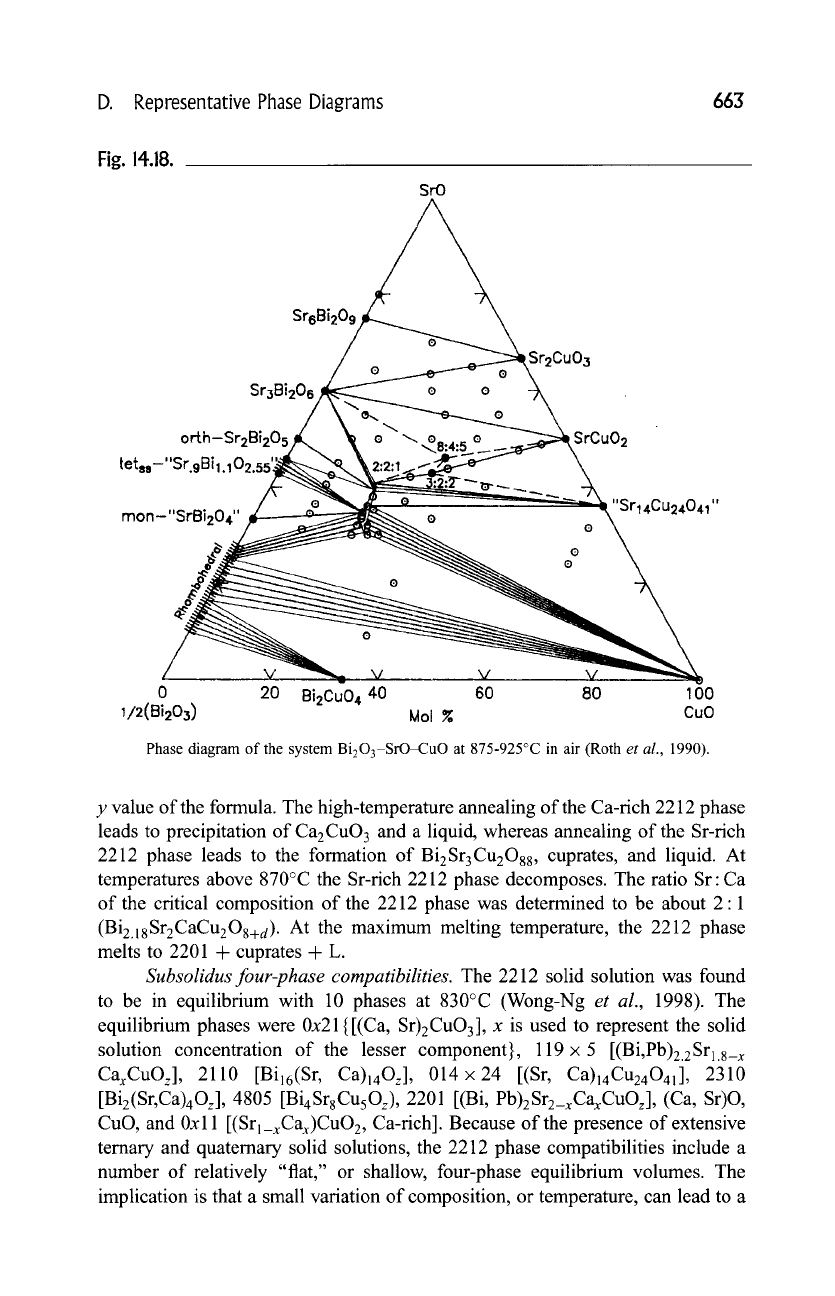
D. Representative Phase Diagrams
663
Fig. 14.18.
SrO
Sr6Bi209
Sr3Bi20s
orth-Sr2Bi205
t et~,-" Sr.9 Bi ~. 102.5s'~
o
mon-"SrBi204" ~ o
0 0
Sr2Cu03
SrCu02
o
o
"Sr14Cu24041"
0 20 Bi2CuO 4 40 60 80 100
1/2(Bi203) Moi ~ CuO
Phase diagram of the system Bi203-SrO-CuO at 875-925~ in air (Roth
et al.,
1990).
y value of the formula. The high-temperature annealing of the Ca-rich 2212 phase
leads to precipitation of CazCuO 3 and a liquid, whereas annealing of the Sr-rich
2212 phase leads to the formation of Bi2Sr3Cu2Os8, cuprates, and liquid. At
temperatures above 870~ the Sr-rich 2212 phase decomposes. The ratio Sr:Ca
of the critical composition of the 2212 phase was determined to be about 2:1
(Biz.18Sr2CaCuzO8+d). At the maximum melting temperature, the 2212 phase
melts to 2201 4- cuprates 4- L.
Subsolidus four-phase compatibilities.
The 2212 solid solution was found
to be in equilibrium with 10 phases at 830~ (Wong-Ng
et al.,
1998). The
equilibrium phases were 0x21 {[(Ca, Sr)zCuO3], x is used to represent the solid
solution concentration of the lesser component}, l19x 5 [(Bi,Pb)z.zSrl.8_ x
CaxCuOz], 2110 [Bi16(Sr, Ca)14Oz], 014x24 [(Sr,
Ca)14Cu24041],
2310
[Biz(Sr, Ca)4Oz], 4805 [Bi4SrgCusOz), 2201 [(Bi,
Pb)zSrz_xCaxCuOz],
(Ca, Sr)O,
CuO, and 0xl 1 [(Srl_xCax)CuO2, Ca-rich]. Because of the presence of extensive
ternary and quaternary solid solutions, the 2212 phase compatibilities include a
number of relatively "flat," or shallow, four-phase equilibrium volumes. The
implication is that a small variation of composition, or temperature, can lead to a
664 Chapter 14: Phase Diagrams
Fig. 14.19.
0.5
1.0
Co
2.5
1.5
/ .,~_~-__-.~-t- ~,... b '"
2...... >
,, I ,,,
! i
1.5 2.0 2.5
Sr
Summary of various determinations of the single-phase solid solution region of the 2212 phase
in the Bi203-SrO-CaO-CuO system (Knizek
et al.,
1993). The cross-section shown
corresponds to the formula
Bi2+ySr3_y_xCaxCu208+,~.
Knizek
et al.
(1993), (e 9 9 850~
air; Holesinger
et al.
(1993), ( .... ) 865~ oxygen; Majewski (1994), (----) 850~ air;
Hong and Mason (1991) (------) 860~ air; Golden
et al.
(1991), (---) varying
temperature; Muller
et al.
(1992), (--) 830~ air.
dramatic change in the phase equilibrium assemblage. Table 14.3 lists a series of
16 four-phase equilibrium volumes arranged according to the initial melting
temperatures obtained from the DTA events (Wong-Ng
et al.,
1998). They are
mutually stable in a topologically consistent manner (i.e., no compositional
overlaps with each other), and they form a close-packed arrangement. The
eutectic temperature (~825~ or the lowest temperature at which the 2212
phase is in equilibrium with liquid, was found to involve a liquid near the
strontium-poor part of the Bi-Sr-Ca-Cu oxide system: 2110 + 119x5 +
2212 + CuO--+ L.
Primary crystallization field of the 2212 phase.
Table 14.3 also shows the
initial melt compositions for the 16 four-phase volumes involving the 2212 phase.
The Bi concentration was found to cover a range from 24 to 42%, Sr from 7 to
33%, Ca from 2 to 27%, and Cu from 19 to 43%. The three-dimensional
pictorialization of the 2212 crystallization volume is shown via the two views in
D.
Representative Phase Diagrams 665
Fig. 14.20.
900
850
800
750
700
- [
//
- I //////
650~~
0.4 0.8 1.2
y in Bi2.18Sr3_yC~u201~
----r I r 1 T I r A
:__..._
2201 + Cuprotee
4- Liquid . .
7
- t~-- 2212+
t \ *,~.~._~.. ~ e.~0~ /
"-/ \:
~ -.t
2CuO3 +
1 ,.---"l
/I
/
2212 + 2212
/
Sr14Cu24041--= /
/
+ s,'~o /
- + 2201
/ -
/
/
/
- ,/ -
/
/
2212 +
-
Co~uO~
+
sr~i~o=
+ CuO
-
1.6 2.0
Temperature-(Sr, Ca) stoichiometry phase relations for the 2212 phase with composition
Bi2.18Sr3_yCayCu208+ 6. The designation 2201 is used to represent the Raveau superconduct-
ing phase (Majewski
et al.,
1995b) Table 14.3 gives initial melts of four-phase equilibrium
volumes containing the 2212 phase.
Figs. 14.21 and 14.22, where approximate temperatures of the four-phase initial
melting equilibria are also indicated. In these diagrams the volumes are expressed
in Cartesian coordinates. The polygonal areas on the surface of the crystallization
volume have been labeled according to the presence of a second solid in
equilibrium with 2212. The maximum melting temperature in Fig. 14.21 is
889~ corresponding to the initial melting equilibrium for the four-phase volume
2212-2310-0x21-CaO. The overall wedgelike shape of the volume is apparent in
Fig. 14.22. In the lower-melting region (low SrO), the volume is very thin and
terminates along a sharp edge. The higher SrO end of the volume is much wider
and is terminated by the 2201 crystallization surface. Inside the volume, the 2212
phase is in equilibrium with liquid. At the comers, it is in equilibrium with three
phases and with L. At the edges, 2212 is in equilibrium with two phases plus L,
and on each face 2212 is in equilibrium with L and the labeled phase.
Effect of Ag addition.
The use of silver as an additive is widespread in
BSCCO tape and wire processing. Except at very high temperaures, the presence
of Ag does not affect the stability of the 2212 phase (Driscoll
et al.,
1993). Figure
14.23 shows a schematic temperature-composition phase relations of the
