Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


Hormonal Regulation of Liver Cytochrome P450 Enzymes
355
cell surface GHR abundance may, at least in part,
be due to differential effects of intermittent vs
continuous GH stimulation^^^ and could play a
role in the activation of distinct intracellular sig-
naling pathways by chronic (female) as compared
to intermittent (male) GH stimulation.
4.2.3.1.
Significance of GH pulse
fi^equency. Studies have been carried out to deter-
mine which of the three descriptive features of a
GH pulse—^namely, GH pulse duration, GH pulse
height, and GH pulse frequency—is required for
proper recognition of a GH pulse as "masculine."
Direct measurement of the actual plasma GH
profiles achieved when GH is administered to
hypophysectomized rats by twice daily s.c. GH
injection
(i.e.,
the intermittent GH replacement pro-
tocol most commonly used to stimulate CYP2C11
expression) has revealed broad peaks of circulating
GH, which last as long as 5-6 hr^^. These sustained
GH "pulses" are effective in stimulating expression
of the male-specific CYP2C11, provided that they
are not administered in close succession. It is thus
apparent that physiological GH pulse duration (<2
hr) is not required to elicit a male CYP response.
Studies carried out in GH-deficient rat models
(either dwarf rats or rats depleted of adult circulat-
ing GH by neonatal MSG treatment) demonstrate
that GH pulse height is also not a critical factor for
stimulation of CYP2C11 expression^^^'
^^^.
This
finding can be understood in terms of the K^ of
the GH-GHR complex, which at 10"^^ M (~2
ng/ml)^^^,
is only 1% of
the
peak plasma hormone
level in adult male rats. In contrast, GH pulse fre-
quency is a critical determinant for GH stimulation
of a male pattern of liver P450 expression, as
shown in hypophysectomized rats given physiolog-
ical replacement doses of GH for 7 days by inter-
mittent intravenous injections at frequencies of 2,4,
6, or 7 times per
day^^.
Analysis of liver CYP2C11
levels in these rats revealed a normal male pattern
of liver CYP2C11 gene expression in response to 6
GH pulses per day (which approximates the normal
male plasma GH pulse frequency), as well as in
response to GH pulses given at lower frequencies, 2
or 4 times per day (e.g., Figure 9.3). However,
hypophysectomized rats are not masculinized by
7 daily GH pulses, indicating that the hepatocyte
does not recognize the pulse as "masculine" if GH
pulsation becomes too frequent. Hepatocytes thus
require a minimum GH off time (—2.5 hr in the
hypophysectomized rat model used in these
studies), which implies the need for an obligatory
recovery period to effectively stimulate CYP2C11
expression. This condition is not met in the case of
hepatocytes exposed to GH continuously (female
hormone profile). This recovery period may serve
to reset the cellular signaling apparatus, for exam-
ple,
by replenishing GHRs at the cell surface (see
below).
4.2.3.2. RoleofGH receptor (GHR). The
effects of GH on hepatocytes and other responsive
cells are transduced by GHR, a 620 amino acid
cell surface transmembrane protein^^^ belonging
to the cytokine receptor superfamily^^"^. GHR is
comprised of a 246 amino acid extracellular
domain that binds GH, a single transmembrane
segment, and a 350 amino acid intracellular
domain that participates in the intracellular signal-
ing events stimulated by GH^^^'
^^^.
X-ray crystal-
lographic and other studies establish that a single
molecule of GH binds in a stepwise manner to
two GHR molecules to yield receptor dimers:
+GHR
GH + GHR -> GH-GHR -> GH-(GHR)2
^^^^
^^7
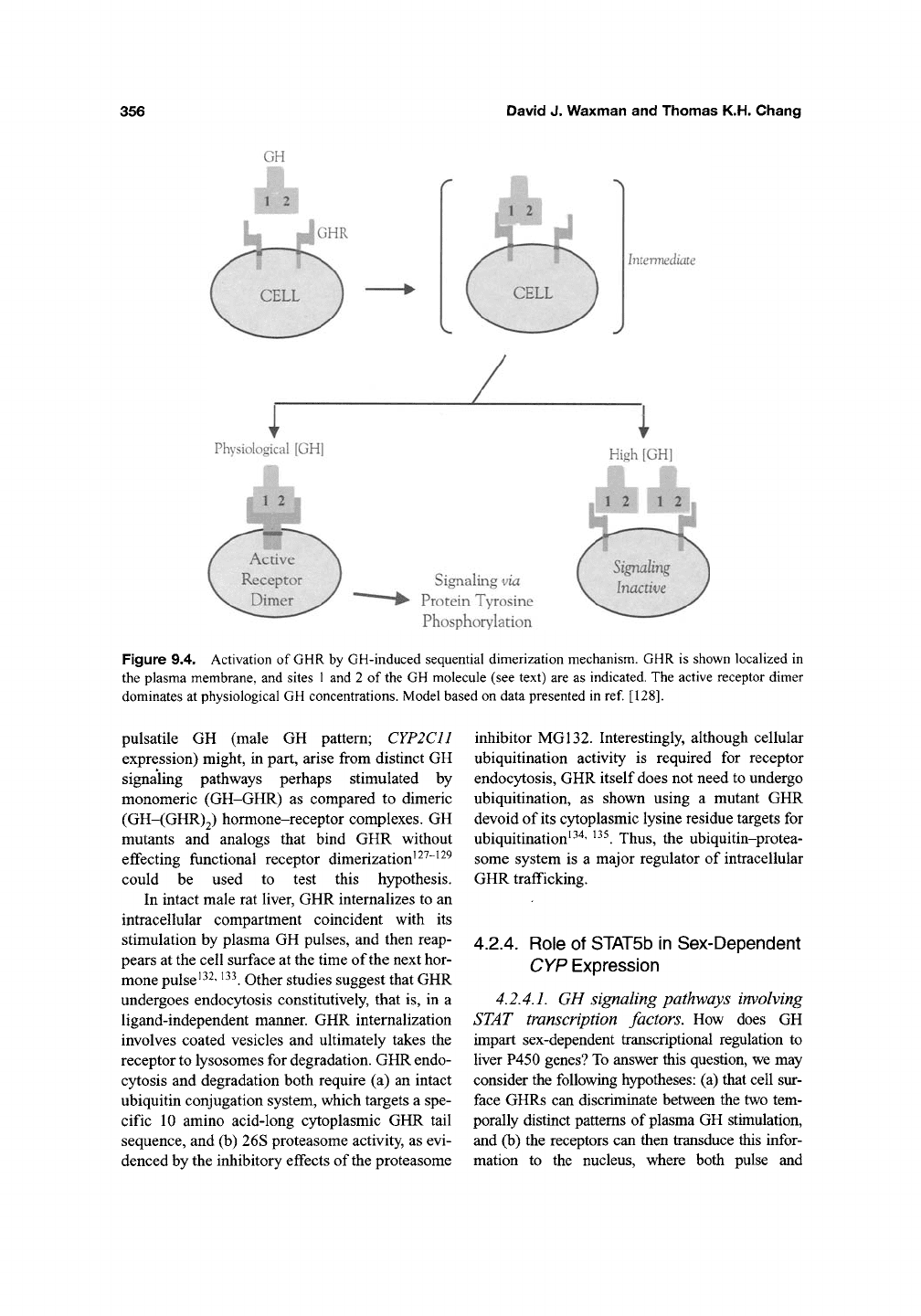
(Figure 9.4).
The four helix bundle protein GH is proposed
to initially bind to a single receptor molecule by
contacts that involve amino acids comprising
GH's Site 1, followed by binding of a second
molecule of GHR, which interacts with Site 2
on the GH molecule to give a heterotrimeric
GH-(GHR)2 complex. GH-induced receptor
dimerization is reversible, and the equilibrium
may be shifted in favor of monomer formation
in the continued presence of excess GH:
CTH
GH-(GHR)2^2GH-GHR. Other, more recent
studies suggest that GH may bind to, and thereby
activate a preformed receptor dimer at the cell sur-
face ^^^' ^^^. Independent of whether the receptor
dimer is preformed, however, it is clear that the
conformational changes that accompany forma-
tion of the active GH-(GHR)2 complex are neces-
sary, and probably sufficient, for stimulation of
GH-induced intracellular signaling events ^^^
(Figure 9.4). Although GHR dimerization is thus
required for most GH responses, some GH
responses might not require receptor dimeriza-
tion^^ ^ and indeed, might be mediated by
monomeric GH-GHR complexes. Conceivably,
the distinct patterns of liver P450 gene expression
induced by continuous plasma GH (female GH
pattern; CYP2C12 expression) as compared to
356
David J. Waxman and Thomas K.H. Chang
GHR
i
Physiological [GH]
Intermediate
i
High [GH]
Signaling via
Protein Tyrosine
Phosphorylation
Figure 9.4. Activation of
GHR
by GH-induced sequential dimerization mechanism. GHR is shown localized in
the plasma membrane, and sites
1
and 2 of
the
GH molecule (see text) are as indicated. The active receptor dimer
dominates at physiological GH concentrations. Model based on data presented in ref
[128].
pulsatile GH (male GH pattern; CYP2C11
expression) might, in part, arise from distinct GH
signaling pathways perhaps stimulated by
monomeric (GH-GHR) as compared to dimeric
(GH-(GHR)2) hormone-receptor complexes. GH
mutants and analogs that bind GHR without
effecting fiinctional receptor dimerization'^^~^^^
could be used to test this hypothesis.
In intact male rat liver, GHR internalizes to an
intracellular compartment coincident with its
stimulation by plasma GH pulses, and then reap-
pears at the cell surface at the time of the next hor-
mone pulse'^^' '^^. Other studies suggest that GHR
undergoes endocytosis constitutively, that is, in a
ligand-independent manner. GHR internalization
involves coated vesicles and ultimately takes the
receptor to lysosomes for degradation. GHR endo-
C5^osis and degradation both require (a) an intact
ubiquitin conjugation system, which targets a spe-
cific 10 amino acid-long cytoplasmic GHR tail
sequence, and (b) 26S proteasome activity, as evi-
denced by the inhibitory effects of the proteasome
inhibitor IVIG132. Interestingly, although cellular
ubiquitination activity is required for receptor
endocytosis, GHR itself does not need to undergo
ubiquitination, as shown using a mutant GHR
devoid of
its
cytoplasmic lysine residue targets for
ubiquitination'^''' '^^. Thus, the ubiquitin-protea-
some system is a major regulator of intracellular
GHR trafficking.
4.2.4.
Role of STAT5b in Sex-Dependent
CYP Expression
4.2.4.1.
GH signaling pathways involving
STAT transcription factors. How does GH
impart sex-dependent transcriptional regulation to
liver P450 genes? To answer this question, we may
consider the following hypotheses: (a) that cell sur-
face GHRs can discriminate between the two tem-
porally distinct patterns of plasma GH stimulation,
and (b) the receptors can then transduce this infor-
mation to the nucleus, where both pulse and
Hormonal Regulation of Liver Cytochrome P450 Enzymes
357
continuous GH-stimulated transcriptional events
occur. Presumably, GH-activated GHR accom-
plishes this by activating two distinct pathways of
intracellular signaling, one in response to GH pulses
and the other in response to continuous GH stimu-
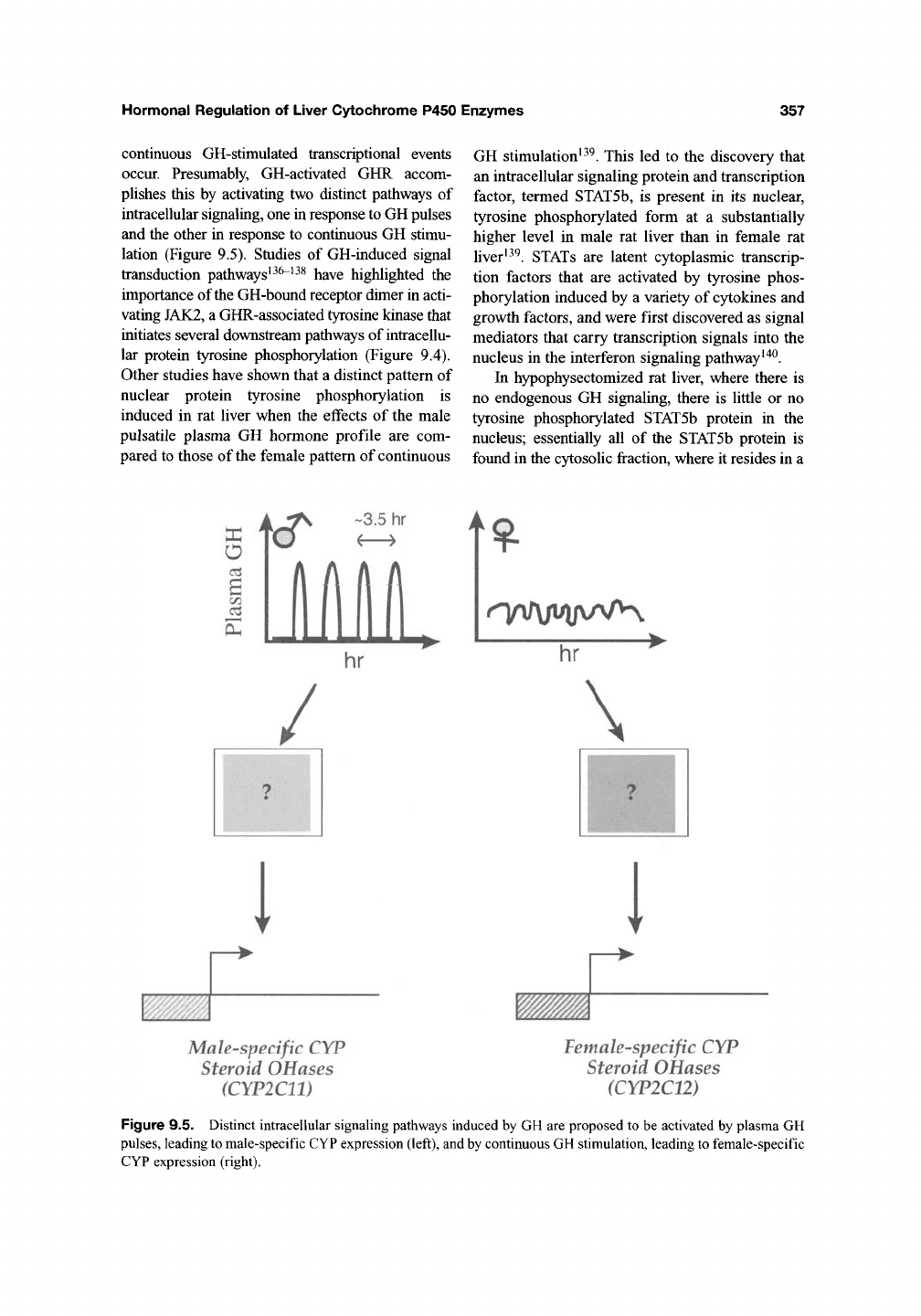
lation (Figure 9.5). Studies of GH-induced signal
transduction pathways^^^^^^ have highlighted the
importance of the GH-bound receptor dimer in acti-
vating JAK2, a GHR-associated tyrosine kinase that
initiates several downstream pathways of intracellu-
lar protein tyrosine phosphorylation (Figure 9.4).
Other studies have shown that a distinct pattern of
nuclear protein tyrosine phosphorylation is
induced in rat liver when the effects of the male
pulsatile plasma GH hormone profile are com-
pared to those of the female pattern of continuous
GH stimulation^^^. This led to the discovery that
an intracellular signaling protein and transcription
factor, termed STATSb, is present in its nuclear,
tyrosine phosphorylated form at a substantially
higher level in male rat liver than in female rat
liver^^^. STATs are latent cytoplasmic transcrip-
tion factors that are activated by tyrosine phos-
phorylation induced by a variety of cytokines and
growth factors, and were first discovered as signal
mediators that carry transcription signals into the
nucleus in the interferon signaling pathway^"^^.
In hypophysectomized rat liver, where there is
no endogenous GH signaling, there is little or no
tyrosine phosphorylated STATSb protein in the
nucleus; essentially all of the STAT5b protein is
found in the C3^osolic fraction, where it resides in a
3.5 hr
I
Male-specific CYP
Steroid OHases
(CYP2C11)
hr
I
Female-specific CYP
Steroid OHases
(CYP2C12)
Figure 9.5. Distinct intracellular signaling pathways induced by GH are proposed to be activated by plasma GH
pulses, leading to male-specific CYP expression (left), and by continuous GH stimulation, leading to female-specific
CYP expression (right).
358 David J. Waxman and Thomas K.H. Chang
latent, inactive (non-tyrosine phosphorylated)
form. However, when a hypophysectomized rat is
injected with a single pulse of GH, STATSb protein
appears in the nucleus in its active, tyrosine phos-
phorylated state within 10-15 min^^^'
^"^^
This tyro-
sine phosphorylation reaction occurs on STATSb
tyrosine residue 699, enabling two STATSb mole-
cules to dimerize via mutual interactions between
the phosphotyrosine residue on one STATSb mole-
cule and the SH2 domain (a protein module that
recognizes and binds specifically to phosphotyro-
sine residues) on a second STATSb molecule. The
STATSb-STATSb dimer that is thus formed quickly
enters the nucleus, where it binds with high affinity
to DNA sites upstream of genes that are transcrip-
tionally activated in response to the initial GH
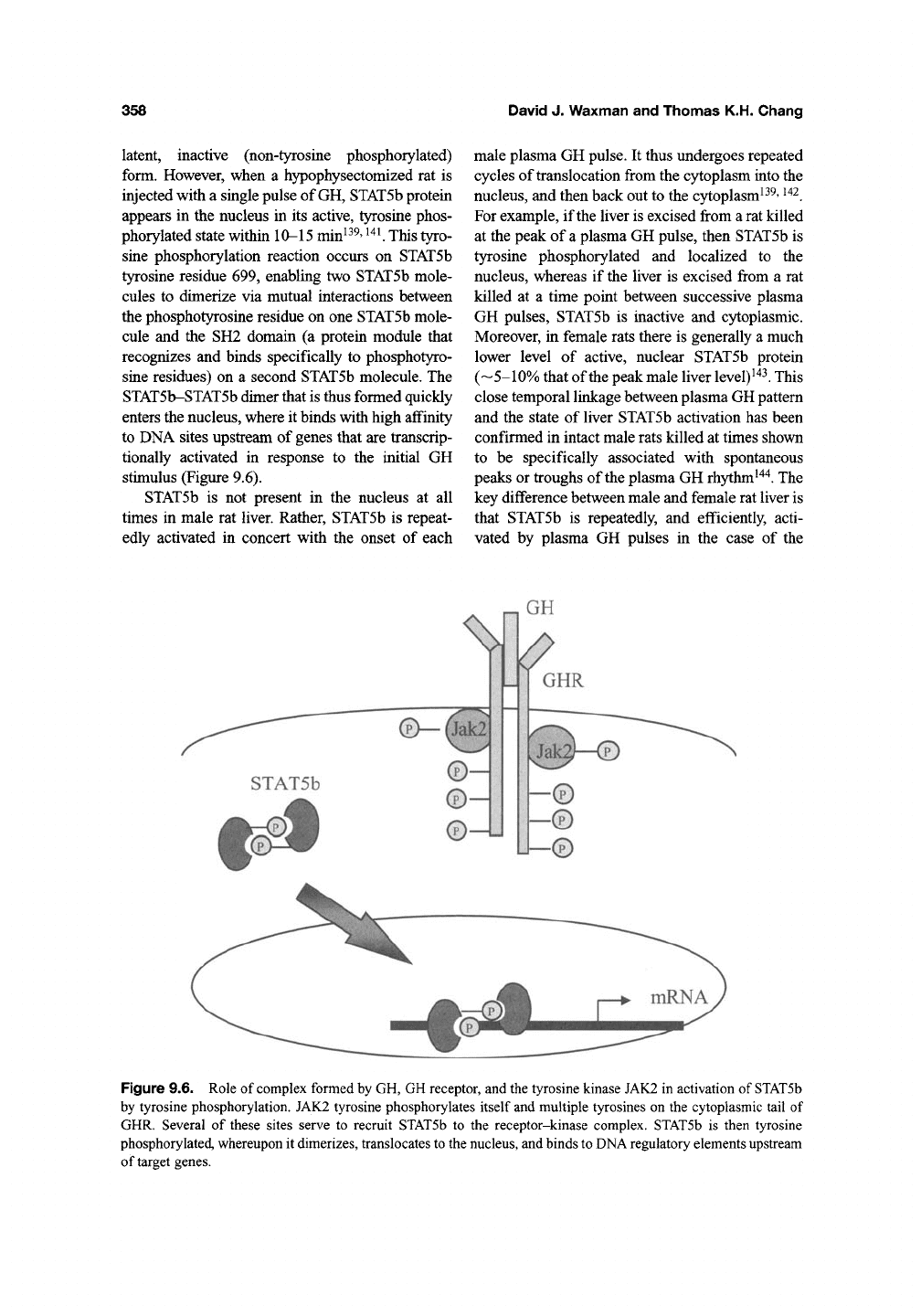
stimulus (Figure 9.6).
STATSb is not present in the nucleus at all
times in male rat liver. Rather, STATSb is repeat-
edly activated in concert with the onset of each
male plasma GH pulse. It thus undergoes repeated
cycles of translocation from the cytoplasm into the
nucleus, and then back out to the cytoplasm^^^' ^'^^.
For example, if the liver is excised from a rat killed
at the peak of
a
plasma GH pulse, then STATSb is
tyrosine phosphorylated and localized to the
nucleus, whereas if the liver is excised from a rat
killed at a time point between successive plasma
GH pulses, STATSb is inactive and cytoplasmic.
Moreover, in female rats there is generally a much
lower level of active, nuclear STATSb protein
(~S-10% that of the peak male liver level)
^'^^.
This
close temporal linkage between plasma GH pattern
and the state of liver STATSb activation has been
confirmed in intact male rats killed at times shown
to be specifically associated with spontaneous
peaks or troughs of the plasma GH
rhythm^"^"^.
The
key difference between male and female rat liver is
that STATSb is repeatedly, and efficiently, acti-
vated by plasma GH pulses in the case of the
GH
Figure 9.6. Role of complex formed by GH, GH receptor, and the tyrosine kinase JAK2 in activation of STATSb
by tyrosine phosphorylation. JAK2 tyrosine phosphorylates itself and multiple tyrosines on the cytoplasmic tail of
GHR. Several of these sites serve to recruit STATSb to the receptor-kinase complex. STATSb is then tyrosine
phosphorylated, whereupon it dimerizes, translocates to the nucleus, and binds to DNA regulatory elements upstream
of target genes.
Hormonal Regulation of Liver Cytochrome P450 Enzymes
359
males,
but is inefficiently activated by the more
continuous GH profile of the females. The rela-
tively weak STATSb activation pathway in female
rat liver appears to reflect a partial desensitization
of this signaling pathway in response to the chronic
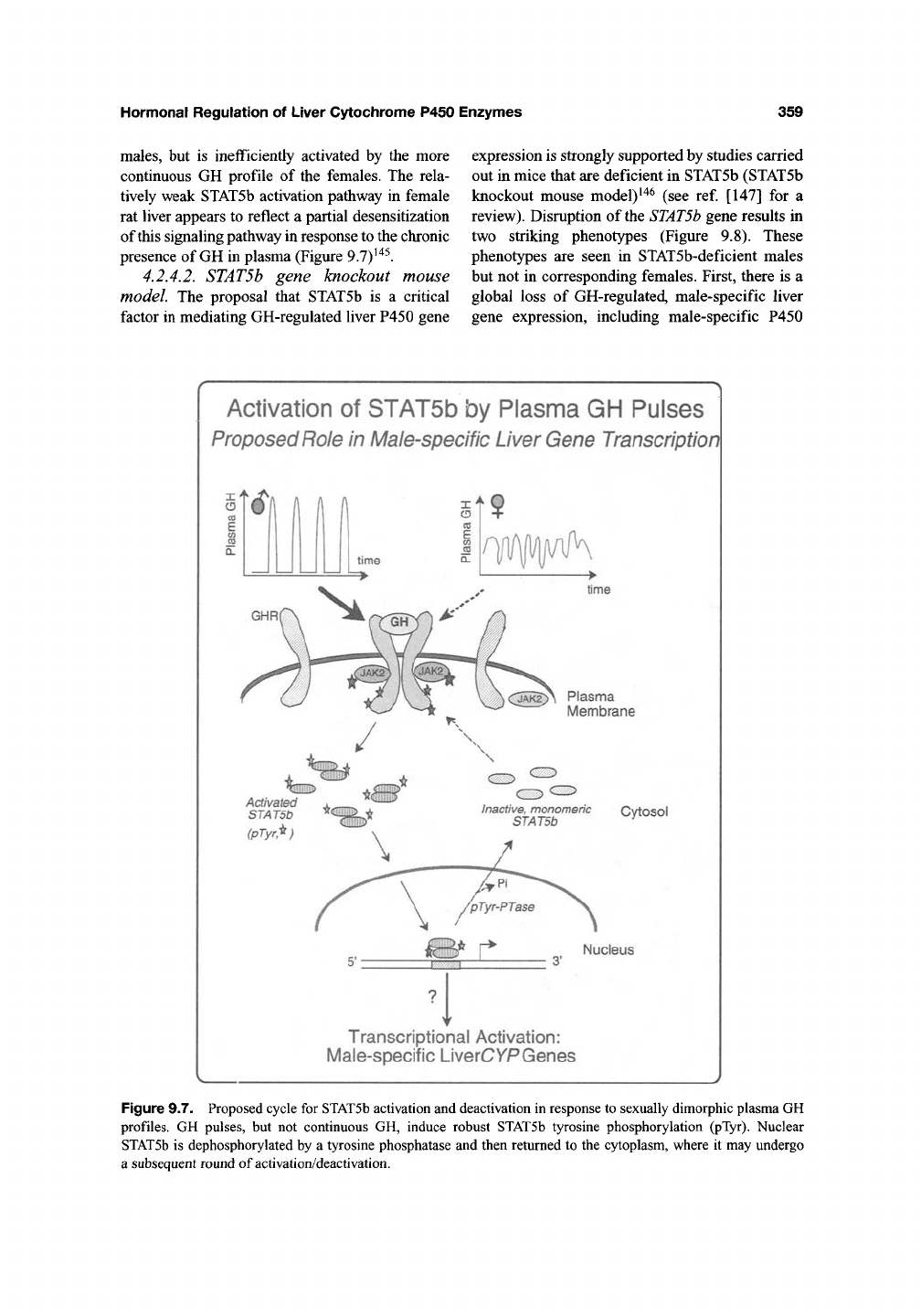
presence of GH in plasma (Figure 9.7)^'*^.
4.2.4.2. STATSb gene knockout mouse
model. The proposal that STATSb is a critical
factor in mediating GH-regulated liver P450 gene
expression is strongly supported by studies carried
out in mice that are deficient in STAT5b (STATSb
knockout mouse model) ^"^^ (see ref [147] for a
review). Disruption of the STAT5b gene results in
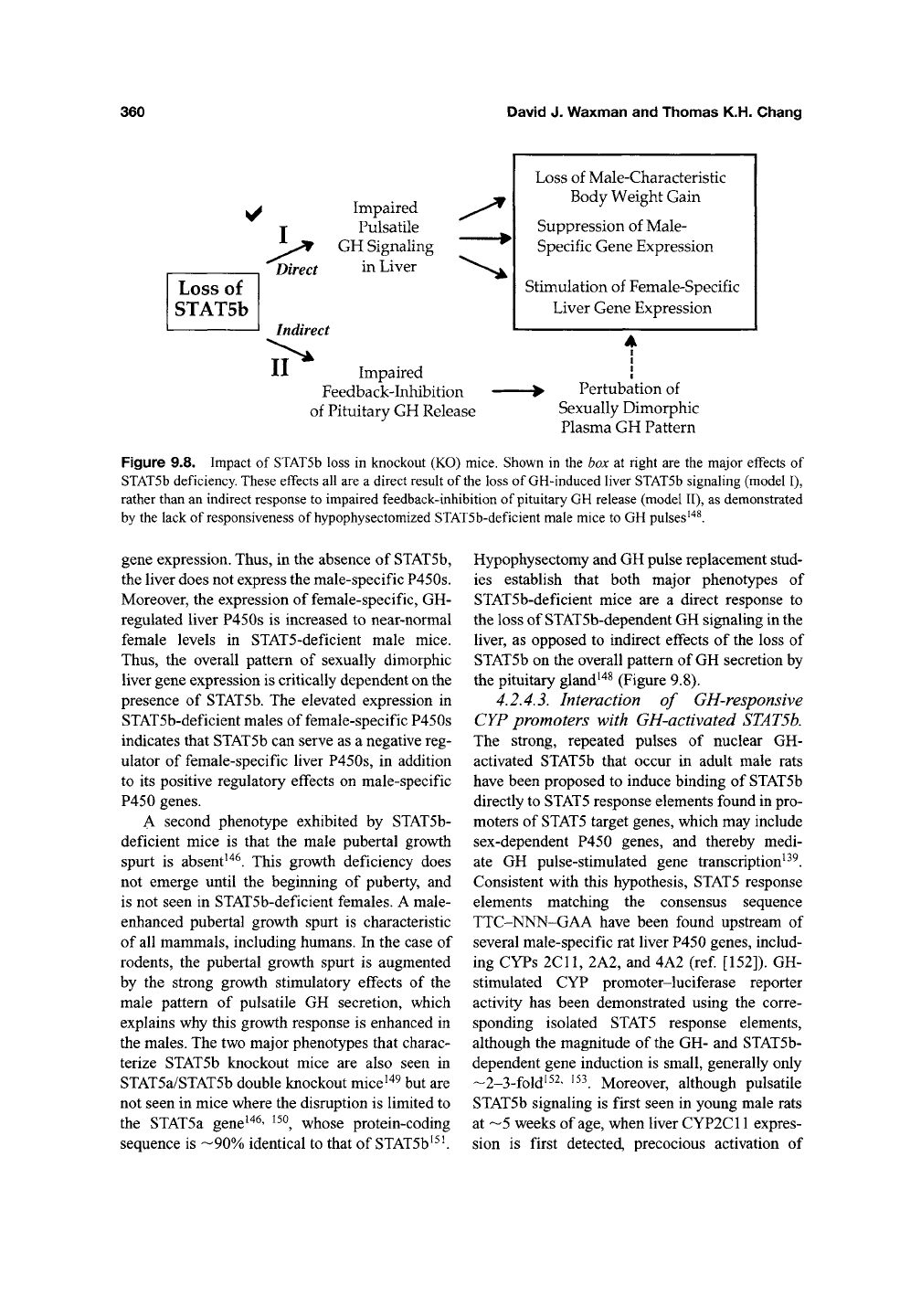
two striking phenotypes (Figure 9.8). These
phenotypes are seen in STATSb-deficient males
but not in corresponding females. First, there is a
global loss of GH-regulated, male-specific liver
gene expression, including male-specific P450
Activation of STATSb by Plasma GH Pulses
Proposed Role in Male-specific Liver Gene Transcriptionl
time
Plasma
Membrane
Cytosol
Nucleus
rm:
Transcriptional Activation:
Male-specific LiverCVP Genes
Figure 9.7. Proposed cycle for STATSb activation and deactivation in response to sexually dimorphic plasma GH
profiles. GH pulses, but not continuous GH, induce robust STAT5b tyrosine phosphorylation (pTyr). Nuclear
STATSb is dephosphorylated by a tyrosine phosphatase and then returned to the cytoplasm, where it may undergo
a subsequent round of activation/deactivation.
360 David J. Waxman and Thomas K.H. Chang
Loss of
STATSb
Direct
Impaired
Pulsatile
GH Signaling
in Liver
Indirect
Loss of Male-Characteristic
Body Weight Gain
Suppression of Male-
Specific Gene Expression
Stimulation of Female-Specific
Liver Gene Expression
II Impaired
Feedback-Inhibition
of Pituitary GH Release
Pertubation of
Sexually Dimorphic
Plasma GH Pattern
Figure 9.8. Impact of STAT5b loss in knockout (KO) mice. Shown in the box at right are the major effects of
STAT5b deficiency. These effects all are a direct result of
the
loss of GH-induced liver STAT5b signaling (model I),
rather than an indirect response to impaired feedback-inhibition of pituitary GH release (model
11),
as demonstrated
by the lack of responsiveness of hypophysectomized STATSb-deficient male mice to GH pulses^^^.
gene expression. Thus, in the absence of STATSb,
the liver does not express the male-specific P450s.
Moreover, the expression of female-specific, GH-
regulated liver P450s is increased to near-normal
female levels in STAT5-deficient male mice.
Thus,
the overall pattern of sexually dimorphic
liver gene expression is critically dependent on the
presence of STATSb. The elevated expression in
STATSb-deficient males of female-specific P4S0s
indicates that STATSb can serve as a negative reg-
ulator of female-specific liver P4S0s, in addition
to its positive regulatory effects on male-specific
P4S0 genes.
A second phenot3^e exhibited by STATSb-
deficient mice is that the male pubertal growth
spurt is absent^"^^. This growth deficiency does
not emerge until the beginning of puberty, and
is not seen in STATSb-deficient females. A male-
enhanced pubertal growth spurt is characteristic
of all mammals, including humans. In the case of
rodents, the pubertal growth spurt is augmented
by the strong growth stimulatory effects of the
male pattern of pulsatile GH secretion, which
explains why this growth response is enhanced in
the males. The two major phenotypes that charac-
terize STATSb knockout mice are also seen in
STATSa/STATSb double knockout
mice^"^^
but are
not seen in mice where the disruption is limited to
the STATS a gene^'*^' ^^^, whose protein-coding
sequence is ~90% identical to that of STATSb^^^
Hypophysectomy and GH pulse replacement stud-
ies establish that both major phenotypes of
STATSb-deficient mice are a direct response to
the loss of STATSb-dependent GH signaling in the
Hver, as opposed to indirect effects of the loss of
STATSb on the overall pattern of
GH
secretion by
the pituitary gland'^^ (Figure 9.8).
4.2.4.3. Interaction of GH-responsive
CYP promoters with GH-activated STATSb.
The strong, repeated pulses of nuclear GH-
activated STATSb that occur in adult male rats
have been proposed to induce binding of STATSb
directly to STATS response elements found in pro-
moters of
STATS
target genes, which may include
sex-dependent P4S0 genes, and thereby medi-
ate GH pulse-stimulated gene transcription^ ^^.
Consistent with this hypothesis, STATS response
elements matching the consensus sequence
TTC-NNN-GAA have been found upstream of
several male-specific rat liver P4S0 genes, includ-
ing CYPs 2C11, 2A2, and 4A2 (ref. [1S2]). GH-
stimulated CYP promoter-luciferase reporter
activity has been demonstrated using the corre-
sponding isolated STATS response elements,
although the magnitude of the GH- and STATSb-
dependent gene induction is small, generally only
~2-3-fold'^^' '^^. Moreover, although pulsatile
STATSb signaling is first seen in young male rats
at ~S weeks of
age,
when liver CYP2C11 expres-
sion is first detected, precocious activation of

Hormonal Regulation of Liver Cytochrome P450 Enzymes
361
STAT5b, achieved in 3-week-old male rats given
pulsatile GH injections, does not lead to preco-
cious CYP2C11 gene induction^'^^. These and
other findings suggest that STAT5b may in part
act in an indirect manner, by regulating the tran-
scriptional activity of liver-enriched transcription
factors (HNFs, hepatocyte nuclear factors) that
cooperate with STAT5b to control the expression
of sexually-dimorphic liver P450 genes^^^'
i^^-^^e
4.2.4.4. Interactions between STAT5b and
liver transcription factors regulating sex-
specific CYPs. Liver-enriched transcription
factors are key determinants of the liver-specific
expression of many hepatic P450s (ref. [157]),
including sex-dependent P450s. Functional
analysis of sex-specific liver CYP proximal pro-
moters has led to the identification of the liver
transcription factors HNFla and HNF3p as
strong ^ra«5-activators of CYP2C11 (ref. [152]).
By contrast, HNFSp and HNF6 serve as strong
^ra«^-activators of CYP2C12 (refs
[155],
[158]),
with synergistic interactions between these two liver
transcription factors leading to an overall ~300-fold
activation of CYP2C12 promoter activity^ ^^. Other
studies indicate a role for C/EBPa^^^' ^^^ and
HNF4^56 in the regulation of CYP2C12 trans-
cription. Interestingly, two of the factors regulating
CYP2C12 expression, HNF6 and HNF3P, require
GH for maximal expression in liver, with HNF6
being expressed at 2-3-fold higher levels in female
compared to male rat liver^^^. This sex difference in
HNF6 levels is too small to account for the
>50-fold higher level of CYP2C12 in female com-
pared to male liver. Similarly, the STAT5b-respon-
siveness of male CYP promoter-derived regulatory
elements^^^' ^^^ is too weak to account for the
high degree of male-specificity (> 100-fold) that
characterizes male-specific CYP expression.
Accordingly, additional factors and GH regulatory
mechanisms are likely to be required for the sex-
specific pattern of gene expression seen in vivo.
One such mechanism may involve an as yet
uncharacterized "regulator of sex-limitation"
("Rsl"), which has been defined genetically and
appears to repress certain GH-regulated, sex-
dependent liver CYP genes in female mouse liver
in a manner that is independent of GH action^^^.
Another possible mechanism for GH-regulated
sex-specific CYP expression involves interactions
between STAT5b and HNFs. For example, studies
of the CYP2C12 promoter have shown that
STAT5b can substantially block the induction
of promoter activity by HNF3P and HNF6
(ref. [152]). This inhibitory action of male GH
pulse-activated STAT5b on HNF3P- and HNF6-
stimulated CYP2C12 transcription is consistent
with the de-repression (i.e., upregulation) of cer-
tain female-specific, GH-regulated mouse P450
genes seen in male STAT5b-deficient mice^"^^' ^^^.
The synergistic interaction between HNF6 and
HNF3P in stimulating CYP2C12 promoter activ-
ity illustrates how small differences in the levels
of these factors in liver cells in vivo (cf. the 2-3-
fold higher expression of HNF6 in females) might
lead to much larger differences in CYP2C12 gene
expression, particularly when coupled to the
inhibitory action of GH pulse-activated STAT5b
in males. Other studies point to additional com-
plexities, based on the potential of STAT5b to
upregulate CYP2C12 gene transcription via an
upstream pair of
STAT5
response elements^^^, and
the potential role of an inhibitory, COOH-terminal
truncated STAT5 form^^^
4.2.4.5. Downregulation of hepatic
STAT5b signaling. Other issues relating to GH
and the STAT5b signaling pathway that are of cur-
rent research interest include how the cycle of
STAT5b activation is turned off at the conclusion
of each GH pulse, and how STAT5b is subse-
quently returned to the cytoplasm in an inactive
form, where it apparently waits for ~2-2.5 hr until
it can be re-activated by the next pulse of GH
(Figure 9.7)^^^. Another important question is why
robust activation of the STAT5b signaling path-
way is not achieved in female liver. The answer to
these questions may, in part, involve an intriguing
family of inhibitory proteins, referred to as SOCS
and CIS proteins, which turn off signals to various
hormones and cytokines, including GH^^^'
^^^.
In
the case of GH signaling, SOCS proteins bind to
the GHR-JAK2 tyrosine kinase complex,
enabling them to inhibit GH signaling by a com-
plex series of interrelated mechanisms ^^^. One of
these inhibitory factors, CIS, may be induced to a
higher level by the continuous (female) GH pat-
tern than by the pulsatile (male) GH pattern. This
inhibitor of
STAT5b
signaling has been implicated
in the downregulation of GH-induced STAT5b
signaling in liver cells exposed to the female GH
pattem^^^

362 David J. Waxman and Thomas K.H. Chang
4.3.
Regulation by Thyroid
Hormone
4.3.1.
Cytochromes P450
Although GH is the major regulator of specific
liver P450s, thyroid hormone also plays a critical
role.
The major thyroid hormones,
T3
and
T4,
pos-
itively regulate some^^' ^^ but not alP^ of the
female-predominant liver P450 enzymes, while
they negatively regulate several of the male-
specific enzymes^^'^"^ (Table 9.1). These effects
of thyroid hormone are operative at the level of
mRNA expression, and are independent of the
indirect effects that thyroid hormone has on liver
P450 levels as a consequence of its effects on liver
GHRs^^^ and its stimulation of GH gene tran-
scription and GH secretion by the pituitary^^^.
Molecular studies of these effects of thyroid hor-
mone have not been carried out.
animals^'^^. The induction of rat hepatic P450
reductase by thyroid hormone in rats occurs by
transcriptional ^^^ and post-transcriptional mecha-
nisms ^^^ and appears to involve enhanced protein
stability in hyperthyroid rat liver*
^'^.
P450 reductase
levels are also modulated by thyroid hormone sta-
tus in several extrahepatic tissues*^^ Conceivably,
interindividual differences in P450 reductase levels
may occur in response to physiological or patho-
physiological differences in circulating th)Toid hor-
mone levels and could be an important contributory
factor to individual differences in P450 reduc-
tase/c54ochrome P450-catalyzed carcinogen metab-
olism and carcinogen activation reactions.
5. Alteration of Liver P450
Expression by Hormonal
Perturbation
4.3.2.
NADPH-Cytochrome P450
Reductase
Thyroid hormone is also required for full
expression of NADPH-cytochrome P450 reduc-
tase,
a flavoenzyme that catalyzes electron transfer
to all microsomal P450s. P450 reductase is an
obligatory, and oflen rate-limiting electron-transfer
protein that participates in all microsomal P450-
catalyzed drug oxidation and steroid hydroxylase
reactions *^^' '^^. This thyroid hormone dependence
of
P450
reductase enzyme expression is evidenced
by the major decrease (>80% reduction) in liver
microsomal P450 reductase activity and P450
reductase mRNA levels that occurs following
hypophysectomy'^^ or in response to methimazole-
induced hypothyroidism^'''. It is further supported
by the reversal of this activity loss when thyroxine
(T4),
but not GH or other pituitary-dependent fac-
tors,
is given at a physiological replacement
dose'^^'
^^K
Restoration of liver P450 reductase
activity in vivo by T4 replacement also effects a
substantial increase in liver microsomal P450
steroid hydroxylase activities. A similar effect can
be achieved when liver microsomes isolated from
hypophysectomized rats are supplemented with
exogenous, purified P450 reductase, which prefer-
entially stimulates steroid hydroxylation catalyzed
by microsomes prepared from thyroid-deficient
As discussed earlier in this chapter, many,
although not all, liver P450s are under hormone
regulatory controls. An individual's circulating
hormonal profile can, however, be altered under
certain situations, including drug therapy, exposure
to chemicals found in the environment, and disease
states such as diabetes and liver cirrhosis. The
resultant changes in circulating hormone levels or
alterations in hormone secretory dynamics could,
therefore, influence the expression of specific liver
P450s. The following sections describe some of
the factors that are known to cause hormonal per-
turbation and discuss the impact of these changes
on liver P450 expression and on P450-dependent
drug and xenobiotic metabolism and toxicity.
5.1.
Modulation by Drugs
The anticancer drugs cisplatin'^^' ''^^, cyclo-
phosphamide''^^' '^^, and ifosfamide'^^ alter the
profile of P450 enzyme expression in liver and
perhaps other tissues, at least in part due to the hor-
monal perturbations that these cytotoxic agents
induce. Treatment of adult male rats with a single
dose of cisplatin depletes serum androgen, and this
effect persists for up to 28 days after drug adminis-
tration'^^. Serum androgen depletion by cisplatin is
associated with a feminization of hepatic liver
enzyme expression. Thus, cisplatin-treated male
rats have elevated levels of the female-predominant

Hormonal Regulation of Liver Cytochrome P450 Enzymes
363
CYP2A1,
CYP2C7, and steroid 5a-reductase, but
have reduced levels of
the
male-specific CYP2A2,
CYP2C11,
and CYP3A2 (refs
[175],
[176]). The
effects of cisplatin on androgen levels may result
from the drug's action on the testes^^^'
^^^;
however,
effects on the hypothalamus are also suggested to
contribute, both to the observed depletion of circu-
lating testosterone and the resultant alteration in
liver P450 expression^ ^^. Cisplatin treatment of
adult female rats severely decreases circulating
estradiol levels and significantly reduces the
expression of the estrogen-dependent CYP2A21,
CYP2C7, and CYP2C12 (ref [176]).
Serum testosterone is also depleted in adult
male rats treated with cyclophosphamide^^^'
^^^^^^^
or ifosfamide^^^ and this depletion is associated
with feminization of liver enzyme profiles^^^'
^^^
in
a manner similar to that produced by cisplatin.
While endogenous androgen secretion can be
stimulated in cyclophosphamide-treated rats by
the luteinizing hormone analog chorionic
gonadotropin, the resultant increase in serum
testosterone does not reverse the loss of hepatic
CYP2C11 expression^^^. This observation is anal-
ogous to the earlier finding that the suppression of
CYP2C11 by 3,4,5,3',4',5'-hexachlorobiphenyli82
is not causally related to the associated depletion
of serum testosterone ^^^. Consequently, modu-
lation of liver enzyme expression by cyclo-
phosphamide may involve action at the
hypothalamic-pituitary axis, which establishes the
sex-dependent plasma GH profile that in turn dic-
tates the expression of CYP2C11 and other sex-
dependent liver P450 enzymes, as discussed earlier
in this chapter. CYP2C11 can also be suppressed
by other mechanisms, as demonstrated by the
finding that CYP2C11 levels are suppressed by
the anticancer drug l-(2-chloroethyl)-3-cyclo-
hexyl-1-nitrosourea (CCNU; lomustine) with-
out affecting circulating testosterone levels ^^'^.
Conceivably, CCNU may act directly on the hypo-
thalamic-pituitary axis to alter key signaling ele-
ments in the ultradian rhythm of circulating GH.
Other drugs that suppress hepatic CYP2C11
and CYP3A2 levels include chloramphenicols^^
and cyclosporine^^^' ^^^. The effects of chlo-
ramphenicol are strain-specific, occurring in
Sprague-Dawley rats but not in Fischer 344 rats.
Moreover, this suppression is accompanied by a
modest reduction in plasma levels of thyroxine but
not testosterone s^^. GH does not appear to play a
role in the suppression of
CYP2C11
and CYP3A2
by cyclosporine, a drug that does not alter the
plasma GH peak amplitude, number, or dura-
tion^^l Phenobarbital^^' s^^'
^^o,
dexamethasoneS9\
and S-fluorouracil^^^ also reduce hepatic CYP2C11
expression, but the mechanism(s) by which these
effects occur have not been elucidated.
5.2. Modulation by Polycyclic
Aromatic Hydrocarbons
Exposure of adult male rats to polycyclic
aromatic hydrocarbons, including 3-methylcholan-
threne (3MC)i^2, i90, 193^ 2,3,7,8-tetrachlorodi-
benzo-p-dioxin^^"^, anthracene, benz(a)anthracene,
dibenz(a,c)anthracene, dibenz(a,/i)anthracene, and
7,12-dimethylbenz(a)anthracene^^^, leads to decre-
ases in hepatic CYP2C11 protein and activity levels.
In the case of 3MC, this suppression reflects a
decrease in the rate of CYP2C11 transcription^^^.
The hormonal mechanisms by which polycyclic aro-
matic hydrocarbons modulate CYP2C11 expression
are not known, however, 3MC^^^ and 2,3,7,8-tetra-
chlorodibenzo-/7-dioxin^^^ have been reported to
decrease serum testosterone levels. 3MC may inter-
fere with the stimulation of
CYP2C11
expression by
GH^^^, but in a manner that does not involve
STAT5bi99. Interestingly, the extent of CYP2C11
suppression by polycyclic aromatic hydrocarbons
is correlated with Ah receptor-binding affinity and
Ah receptor transformation potency^ ^^. However, it
is not clear whether the Ah receptor plays a role in
the transcriptional suppression of
CYP2C11
by poly-
cyclic aromatic hydrocarbons^^^.
5.3. Modulation by
Pathophysiological State
5.3.1.
Diabetes
Uncontrolled insulin-dependent diabetes is
accompanied not only by defective carbohydrate
metabolism, which results in hyperglycemia,
hyperlipidemia, and hyperketonemia, but it is also
associated with hormonal perturbation, including
a reduction in circulating testosterone^^ ^~^^^,
th3a'oid hormone, and plasma
Gtf^'*'
^^^. As
described earlier in this chapter, these hormones
regulate many liver P450 enzymes, either directly
or indirectly. Accordingly, the diabetic state is

364 David J. Waxman and Thomas K.H. Chang
associated with profound changes in the levels of
various hepatic P450 enzymes. Whereas diabetes
leads to induction of several rat liver P450 forms,
including CYP2A1 (refs
[205],
[206]), CYP2B1
(refs
[207],
[208]), CYP2C7 (ref [206]), CYP2E1
(refs [209]-[212]), CYP4A2 (ref. [206]), and
CYP4A3 (ref [206]), it suppresses CYP2A2 (ref
[205]),
CYP2C11 (refs
[205], [207], [208],
[212]),
and CYP2C13 (ref. [205]). Changes in the levels
of some of these liver P450s (e.g., CYP2C11 and
CYP2E1) have been shown at the mRNA leveP^^,
208,213,214
^jj^
^j.g reversed by insulin replacement.
The profile of GH secretion in the diabetic male
rat is altered so as to resemble the pattern found in
the normal female
rat^^^.
The induction of CYP2A1
and CYP2C7 in diabetic male rats can therefore be
explained, at least in part, as a response to the more
continuous pattern of GH secretion, which stimu-
lates expression of these P450 forms^^'
3U 63,215
jj^
contrast, this pattern of GH secretion reduces
CYP2A2 and CYP2C13 levels because continuous
GH administration suppresses these two
P450s2^'28'
116
CYP2C11 expression is obligatorily
dependent on the intermittent male pattern of
plasma GH secretion^^. Therefore, the more contin-
uous secretion of GH in diabetic male
rats^^'*
would
be expected to suppress this P450. In the case of
CYP2B1,
GH pulse height is the suppressive
signal
^^^
and accordingly, the reduction in GH peak
concentration in diabetic male rats^^^ leads to
increases in CYP2B1 levels^^^' 208 A GH-inde-
pendent mechanism is likely to contribute to some
of the other effects of diabetes on liver P450
expression. GH, independent of
its
plasma profile,
is suppressive toward hepatic CYP2E1 (ref [62]),
but the levels of this P450 are substantially elevated
in both diabetic male and female rats^^^' 216,217
CYP2E1 induction in diabetes has been attributed
to increased plasma concentrations of ketone bod-
jgg2io,
218
Recently, a role of hypoinsulinemia and
hyperglucagonemia was proposed for the suppres-
sion of
CYP2C11
in diabetes, based on the finding
that treatment of cultured rat hepatocytes with
glucagon decreases CYP2C11 expression in a
cAMP-dependent manner and this decrease can be
reversed by insulin administration^ ^^.
5.3.2. Liver Cirrhosis
Gonadal abnormalities occur in liver cirrhosis.
Adult male rats fed a chronic choline-deficient
diet to induce cirrhosis have enhanced serum
estradiol concentrations^^^ and reduced testicular
weight^^^ and serum testosterone levels^^^. In
association with the perturbation in hormonal
status is a major decline in hepatic CYP2C11 con-
tent^^^, and this decline is not accompanied by
induction of steroid 5a-reductase activity^^^. The
suppression of hepatic CYP2C11 is also evident in
other models of liver cirrhosis, including bile duct
ligation^^^' ^^^ and carbon tetrachloride-induced
cirrhosis^^^' ^^^. Whether the alteration in serum
steroid hormone levels is directly or indirectly
responsible for the apparent demasculinization of
liver P450 remains to be determined.
5.4. Modulation by Ethanol and
Dietary Factors
Adult male rats administered ethanol by a total
enteral nutrition system have reduced hepatic
CYP2C11 and CYP3A2 levels, whereas their
CYP2A1 activity is unaltered^^^. The same ethanol
treatment alters the dynamics of plasma GH secre-
tion by decreasing the GH pulse amplitude and
increasing the GH pulse frequency. The increased
frequency of GH pulses thus explains the reduced
expression of CYP2C11 after chronic ethanol
intake because hepatocytes require a minimum "off
time"
in order to express the male-pattern of GH
secretion that stimulates CYP2CI1 expression^^. In
another study, chronic intragastric infusion of
ethanol-containing diets resulted in suppression of
CYP3A2 while substantially increasing the expres-
sion of CYP3A9 in adult male rats^^^
Dietary vitamin-A deficiency reduces hepatic
CYP2C11 (refs [227]-[229]) and CYP4A2
levels^^^,
while inducing steroid 5a-reductase
activity in adult male rats^"^^. These effects, which
are accompanied by a reduction in plasma testos-
terone levels, can be restored by the inclusion of
all-
trans-TQiinoic
acid in the diet^^^ or by exogenous
administration of androgen^^^'
^^K
Interestingly,
twice daily subcutaneous administration of GH,
which is known to induce CYP2C11 expression
in hypophysectomized male rats^^, is not effective
in restoring the levels of
CYP2C11
or CYP4A2 in
male rats fed a vitamin A-deficient diet^^^.
Dietary trace minerals can also alter liver
expression of sex-dependent P450 enzymes.
Prepubertal male rats fed a zinc-deficient diet
