Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.

' Modern Industrial Microbiology and Biotechnology
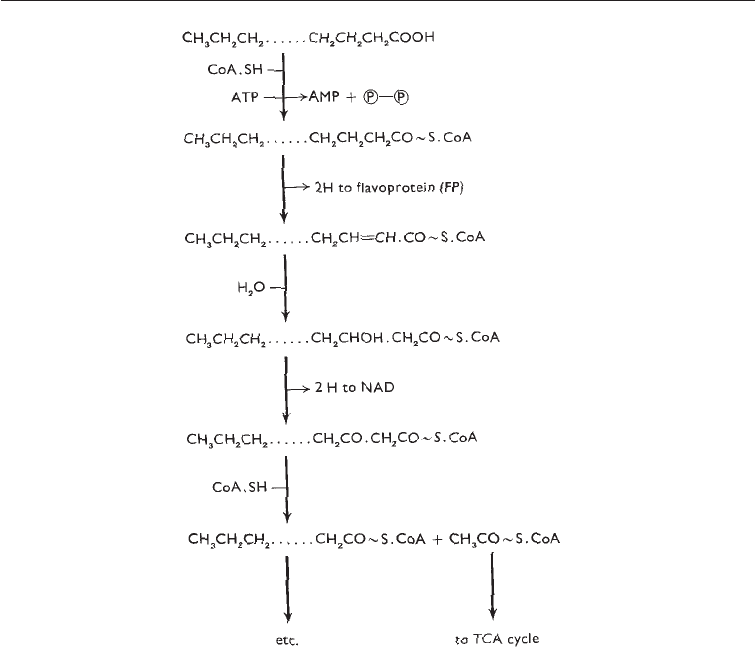
Fig. 5.7 b-oxidation of Fatty Acids
(ii) the assembly of these intermediates in an unusual way, by means of a combination
of standard general mechanisms with a selection from a relatively small number of
special mechanism;
(iii) these special mechanisms while being peculiar to secondary metabolism are not
unrelated to general or primary mechanism;
(iv) the synthetic activity of secondary metabolism appears in response to conditions
favorable for cell multiplication.
From the above, it becomes clear that although secondary metabolites are diverse in
their intrinsic chemical nature as well as in the organism which produce them, they use
only a few biosynthetic pathways which are related to, and use the intermediates of, the
primary metabolic pathways. Based on the broad flow of carbon through primary
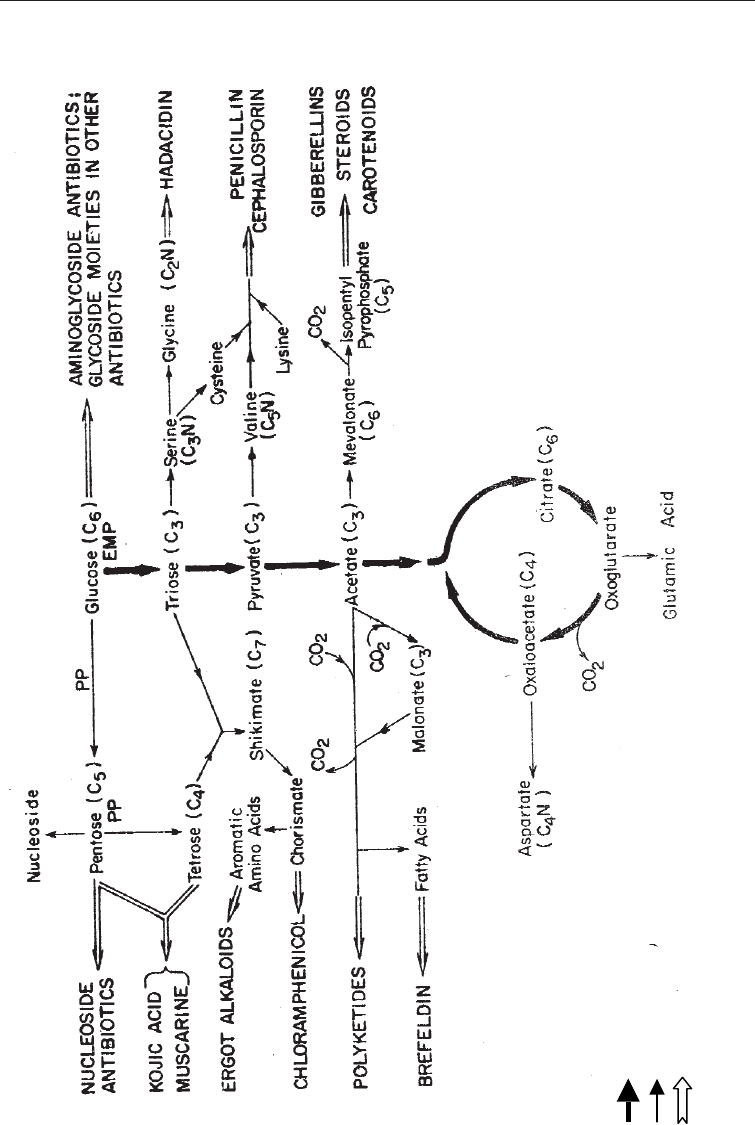
metabolites to secondary metabolites, (depicted in Fig. 5.8) the secondary metabolites
may then be classified according to the following six metabolic pathways.
(i) Secondary products derived from the intact glucose skeleton: The carbon skeleton of
glucose is incorporated unaltered in many antibiotics and other secondary
metabolites. The entire basic structure of the secondary product may be derived
from glucose as in streptomycin or it may form the glycoside molecule to be
combined with a non-sugar (aglycone portion) from another biosynthetic route.
Metabolic Pathways for the Biosynthesis of Industrial Microbiology Products '
Fig. 5.8 Biosynthetic Routes Between Primary and Secondary Metabolites
Main carbon routes
Primary metabolic routes
Secondary metabolic routes
Compounds in heavy type = secondary metabolites.
' Modern Industrial Microbiology and Biotechnology
The incorporation of the intact glucose molecule is more common among the
actinomycetes than among the fungi.
(ii) Secondary products related to nucleosides: The pentose phosphate pathway
provides ribose (5 carbon) for nucleoside biosynthesis. Many secondary
metabolites in this group are antibiotics and are produced mainly by
actinomycetes and fungi. Examples are nucleoside antibiotics such as bleomycin.
(Chapter 21).
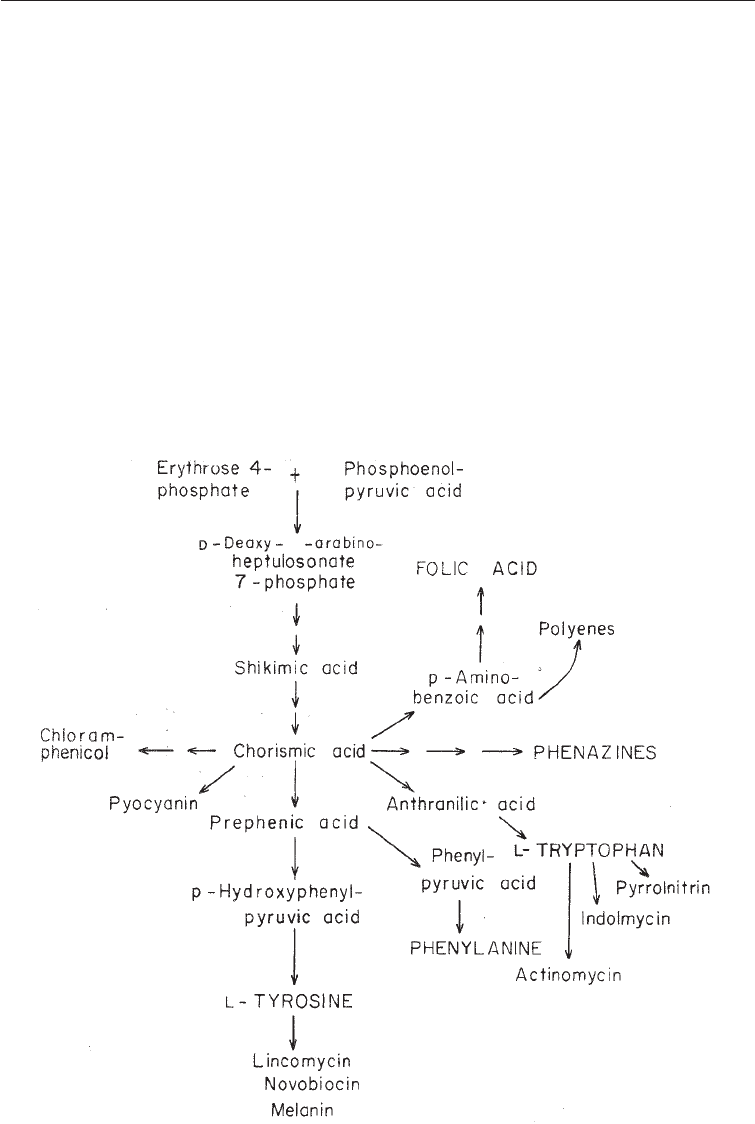
(iii) Secondary products derived through the Shikimate-Chorismate Pathway:
Shikimic acid (C
7
) is formed by the condensation of erythrose-4- phosphate (C
4
)
obtained from the PP pathway with phosphoenolypyruvate (C
3
) from the EMP
pathway. It is converted to chorismic acid which is a key intermediate in the
formation of numerous products including aromatic aminoacids, such as
phynylalamine, tryrosine and tryptophan. Chorismic acid is also a precursor for a
number of secondary metabolites including chloramphenicol, p-amino benzoic
acid, phenazines and pyocyanin which all have anticrobial properties (Fig. 5.9).
The metabolic route leading to the formation of these compounds is therefore
Fig. 5.9 Metabolites in the Shikimic-Chorismate Pathway
Metabolic Pathways for the Biosynthesis of Industrial Microbiology Products '!
referred to as the shikimate pathway. In view of this central role of chorismic acid,
however, the route is more widely known as the shikimate-chorismate route. The
shikimate-chorismate route is an important route for the formation of aromatic
secondary products in the bacteria and actinomycetes. Examples of such
secondary products include chloramphenicol and novobiocin. The route is less
used in fungi, where the polyketide pathway is more common for the synthesis of
aromatic secondary products.
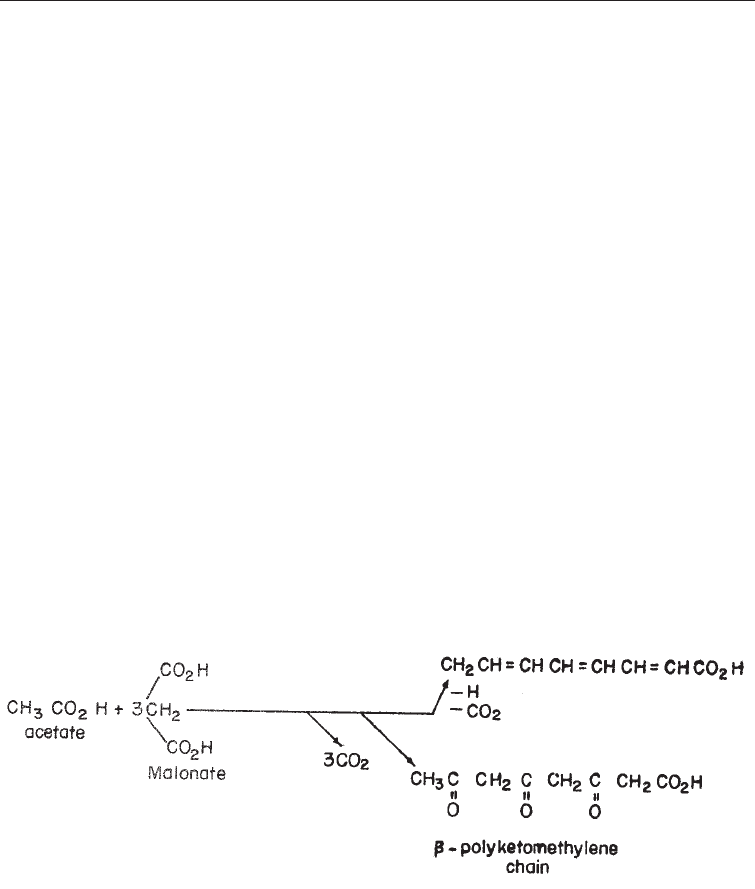
(iv) The polyketide pathway: polyketide biosynthesis is highly characteristic of the
fungi, where more secondary metabolites are produced by it than by any other.
Indeed most of the known polyketide-derived natural products have been obtained
from the fungi, a much smaller number being obtained from bacteria and higher
plants. The triose (C
3
) derived from glucose in the EMP pathway is converted via
pyruvic acid to acetate, which occupies a central position in both primary and
secondary synthesis. The addition of CO
2
to an acetate group gives a malonate
group. The synthesis of polyketides is very similar to that of fatty acids. In the
synthesis of both groups of compounds acetate reacts with malonate with the loss
of CO
2
. By successive further linear reactions between the resulting compound and
malonate, the chain of the final compound (fatty acid or polyketide) can be
successively lengthened.
However, in the case of fatty acid the addition of each malonate molecule is
followed by decarboxylation and reduction whereas in polyketides these latter
reactions do occur. Due to this a chain of ketones or a b-polyketomethylene (hence
the name polyketide) is formed (Fig. 5.10). The polyketide (b - poly-ketomethylene)
Fig. 5.10 Formation of Polyketides
chain made up of repeating C-CH
2
or ‘C
2
units’, is a reactive protein-bound
intermediate which can undergo a number of reactions, notably formation into
rings. Polyketides are classified as triketides, tetraketides, pentaketides, etc.,
depending on the number of ‘C
2
units’. Thus, orsellenic acid which is derived from
the straight chain compound in Fig. 5.11 with four ‘C
2
-units’ is a tetraketide.
Although the polyketide route is not common in actinomycetes, a modified
polyketide route is used in the synthesis of tetracyclines by Streptomyces griseus.
(v) Terpenes and steroids: The second important biosynthetic route from acetate is that
leading via mevalonic acid to the terpenes and steroids. Microorganisms
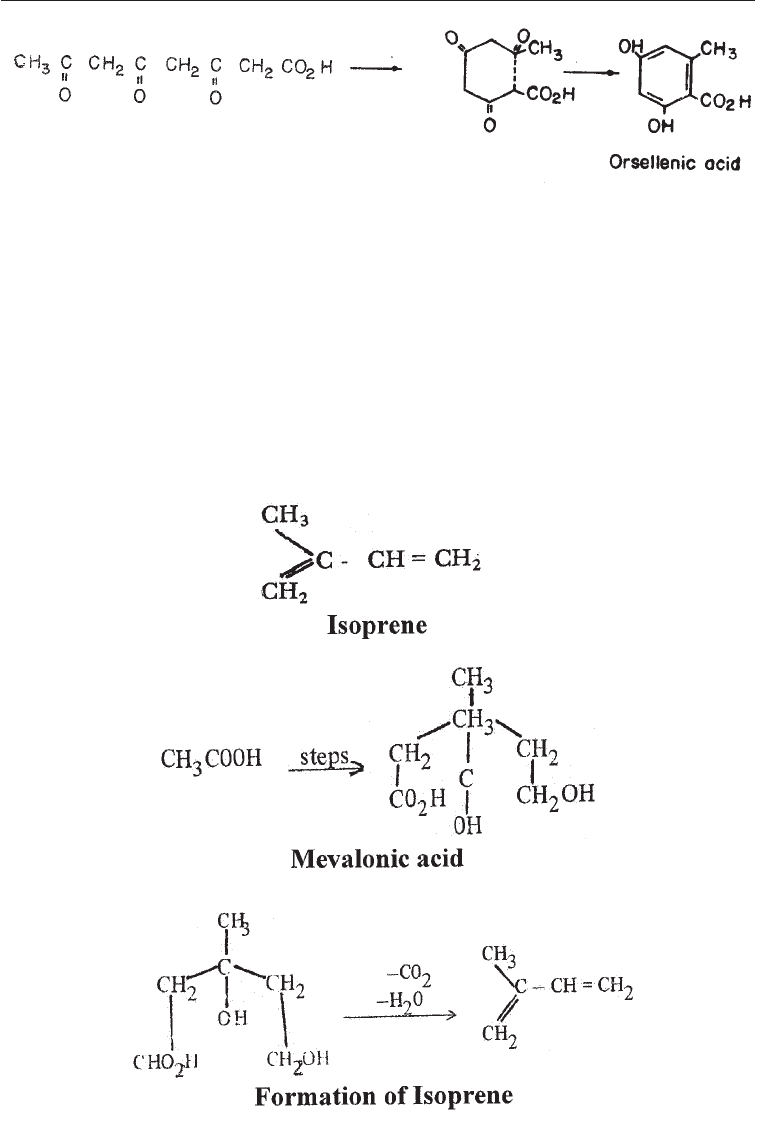
'" Modern Industrial Microbiology and Biotechnology
Fig. 5.12 Isoprene Derivatives
especially fungi and bacteria synthesize a large number of terpenes, steroids,
carotenoids and other products following the ‘isoprene rule’. The central point of
this rule is that these compounds are all derivatives of isoprene, the five-carbon
compound.
Simply put the isoprene rules consist of the following (Fig. 5.12):
(i) Synthesis of mevalonate from acetate or leucine
(ii) Dehydratopm and decarboxylation to give isoprene followed by
condensation to give isoprenes of various lengths.
(iii) Cyclization (ring formation) e.g., to give steroids (Chapter 26)
Fig. 5.11 Formation of the Triketide, Orsellenic Acid
Metabolic Pathways for the Biosynthesis of Industrial Microbiology Products '#
(iv) Further modification of the cyclised structure. The route leads to the
formation of essential steroid hormones of mammals and to a variety of
secondary metabolites in fungi and plants. it is not used to any extent in the
actinomycetes.
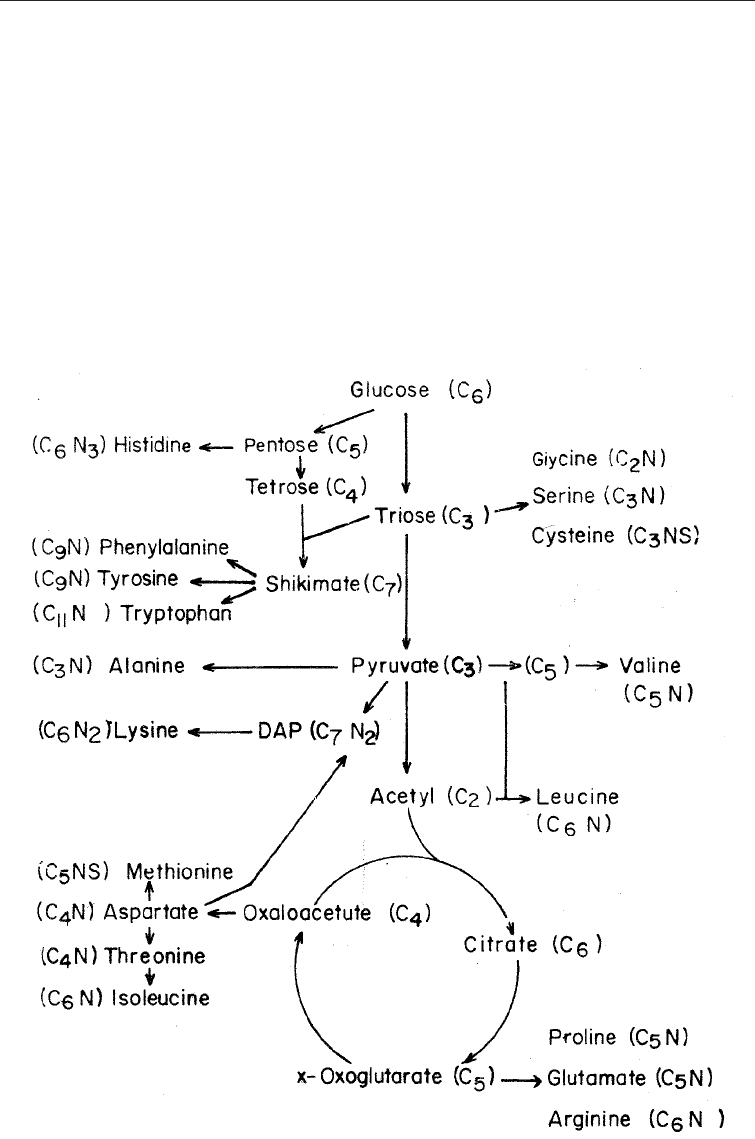
(vi) Compounds derived from amino acids: The amino acids are derived from various
products in the catabolism of glucose. Serine (C
3
N) and glycine (C
2
N) are derived
from the triose (C
3
) formed glucose; valine (C
5
N) is derived from acetate (C
3
);
aspartatic acid (C
4
N) is derived from oxeloacetic acid (C
4
) while glutamic acid
(C
5
N) is derived from oxoglutamic acid (C
5
). The biosynthetic pathways for the
formation of amino acids are shown in Fig. 5.13 from which it will be seen that
aromatic amino acids are derived via the shikimic pathway.
Secondary products may be formed from one, two or more amino acids. an example of
the first group (with one amino acid group) is hadacidin which inhibits plant tumors and
Fig. 5.13 Synthetic Routes of the Amino Acids
'$ Modern Industrial Microbiology and Biotechnology
is produced from glycine and produced by Penicillium frequentants according to the
formula shown below
H
2
NCH
2
CO
2
H ® HN(OH) CH
2
CO
2
H ® OHCN(OH) CH
2
CO
2
H
Glycine Hadacidin
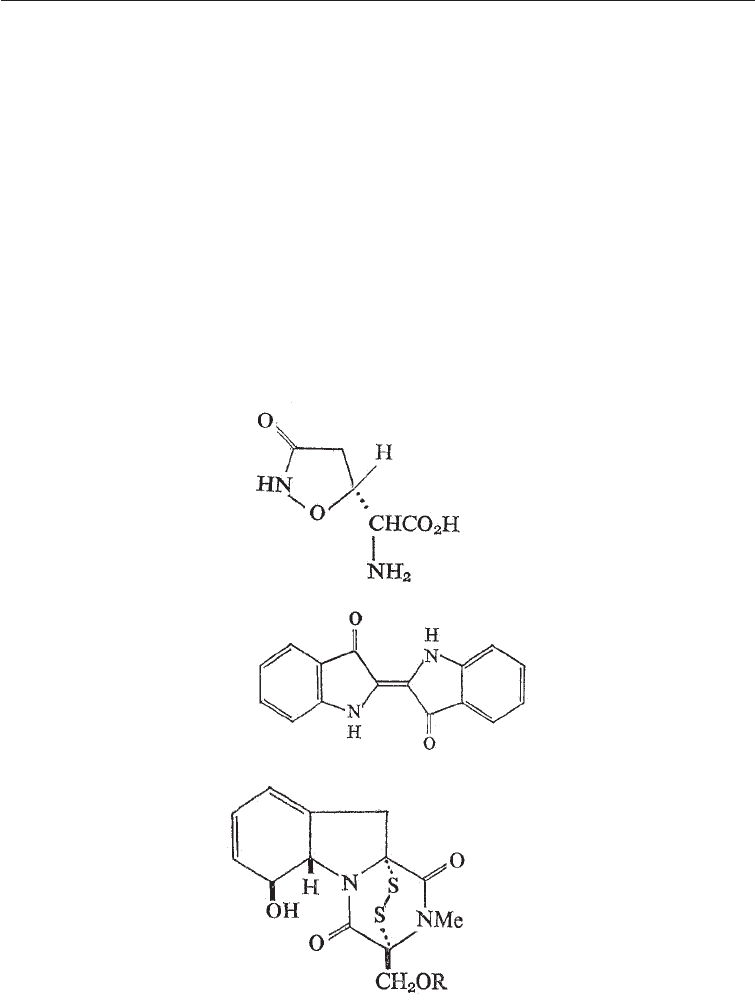
Other examples are the insecticidal compound, ibotenic acid (Amanita factor C)
produced by the mushroom Amanita muscaria and psilocybin, a drug which causes
hallucinations and produced by the fungus Psiolocybe (Fig. 5.14), the ergot alkaloids
(Chapter 25) produced by Clavicepts purpureae also belong in this group as does the
antibiotic cycloserine.
Among the secondary products derived from two amino acids are gliotoxin which is
produced by members of the Fungi Imperfecti, especially Trichoderma and which is a
highly active anti-fungal and antibacterial (Fig. 5.14) and Arantoin, an antiviral drug
also belongs to this group.
Top: Ibotenic acid (from one amino acid)
Middle: Indole (from one amino acid)
Bottom: Gliotoxin (from two amino acids)
Fig. 5.14 Secondary Metabolites Formed from Amino Acids
Metabolic Pathways for the Biosynthesis of Industrial Microbiology Products '%
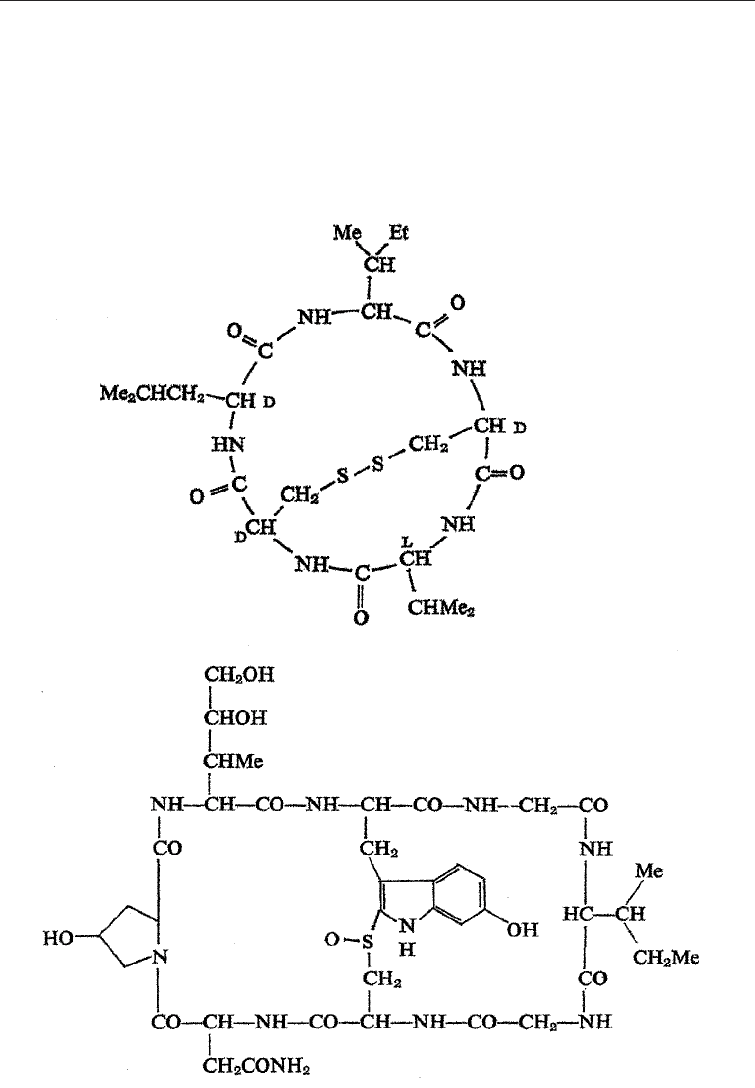
Top: Malformin A, a secondary metabolite formed from three amino acids
Bottom: Amanitin, a secondary metabolite formed from two amino acids
Fig. 5.15 Secondary Metabolites from Amino Acids
The secondary products derived from more than two amino acids include many
which are of immense importance to man. These include many toxins from mushrooms
e.g the Aminita toxins (Fig. 5.15) (phalloidin, amanitin) peptide antibiotics from Bacillus
app and a host of other compounds.
An example of a secondary metabolite produced from three amino acids is malformin
A (Fig. 5.15) which is formed by Aspergillus spp. It induces curvatures of beam shoots and
maize seedlings. It is formed from L-leucine, D-leucine, and cysteine.

'& Modern Industrial Microbiology and Biotechnology
SUGGESTED READINGS
Bull, A.T., Ward, A.C., Goodfellow, M. 2000. Search and Discovery Strategies for Biotechnology:
The Paradigm Shift Microbiology and Molecular Biology Reviews 64, 573 – 606.
Demain, A.L. 1998. Induction of microbial secondary Metabolism International Microbiology 1,
259–264.
Herrmann, K.H., Weaver, L.M. 1999. The Shikimate Pathway. Annual Review of Plant
Physiology and Plant Molecular Biology. 50, 473–503.
Madigan, M.T., Martinko, J.M. 2006. Brock Biology of Micro-organisms. Pearson Prentice Hall
Upper Saddle River, USA.
Meurer, G., Hutchinson, C.R. 1999. Genes for the Synthesis of Microbial Secondary M etabolites.
In: Manual of Industrial Microbiology and Biotechnology. A.L. Demain and J.E. Davies, (eds).
ASM Press. 2
nd
Ed. Washington, DC, USA pp. 740-758.
Zahner, H. 1978. In: Antibiotics and other Secondary Metabolites. R. Hutter, T. Leisenger, J.
Nuesch, W. Wehrli (eds). Academic Press, New York, USA, pp. 1-17.

Overproduction of Metabolites of Industrial Microorganisms 99
The complexity of the activities which go on within a cell was mentioned at the beginning
of Chapter 5 when we discussed the metabolism of a yeast cell introduced into an
aqueous solution of glucose and ammonium salts. The yeast cell must first permit the
entry into itself of the glucose and ammonium salts. Under suitable environmental
conditions such as pH and temperature it will grow by budding within about half an
hour. For these buds to occur, hundreds of activities will have gone on within the cell.
New proteins to be incorporated into enzymes and other structures will have been
synthesized; nucleic acids for the chromosomes and carbohydrates for the cell walls will
all have been synthesized. Hundreds of different enzymes will have participated in these
synthetic activities. The organism must synthesize each of the compounds at the right
time and in the appropriate quantities. If along side ammonium salts, amino acids were
supplied, the yeast cells would stop absorbing the ammonium salt and instead utilize the
supplied ‘readymade’ substrate.
A few yeasts can utilize starch. If our yeast belonged to this group and was supplied
nothing but starch and ammonium salts, it would secrete extracellular enzyme(s) to
breakdown the starch to sugars. These sugars would then be absorbed and would be
used with ammonium salts, for the synthetic activities we described earlier.
Clearly therefore, while the organism’s genetic apparatus determines in broad terms
the organism’s overall synthetic potentialities, what is actually synthesized depends on
what is available in the environment. Most importantly, the organism is not only able to
‘decide’ when to manufacture and secrete certain enzymes to enable it to utilize materials
in the environment, but it is able to decide to stop the synthesis of certain compounds if
they are supplied to it. These sensing mechanisms for the switching on and off of the
synthetic processes enable the organism to avoid the overproduction of any particular
compound. If it did not have these regulatory mechanisms it would waste energy and
resources (which are usually scarce in natural environments) in making materials it did
not require.
An efficient or ’stringent’ organism which does not waste its resources in producing
materials it does not require will survive well in natural environments where competition
Overproduction of
Metabolites of Industrial
Microorganisms
6
CHAPTER
