Matar Sami, Hatch Lewis F. Chemistry of petrochemical processes
Подождите немного. Документ загружается.

acid with ethylene glycol or 1,4-butanediol. These materials are used to
produce film for magnetic tapes due to their abrasion and chemical resis-
tance, low water absorption, and low gas permeability. Polyethylene
terephthalate (PET) is also used to make plastic bottles (approximately
25% of plastic bottles are made from PET). Similar to nylons, the most
important use of PET is for producing synthetic fibers (discussed later).
Polybutylene terephthalate (PBT) is another thermoplastic polyester pro-
duced by the condensation reaction of terephthalic acid and 1,4-butanediol:
Synthetic Petroleum-Based Polymers 337
The polymer is either produced in a bulk or a solution process. It is
among the fastest growing engineering thermoplastics, and leads the
market of reinforced plastics with an annual growth rate of 7.3%.
23
The 1997 U.S. production of thermoplastic polyesters was approxi-
mately 4.3 billion pounds.
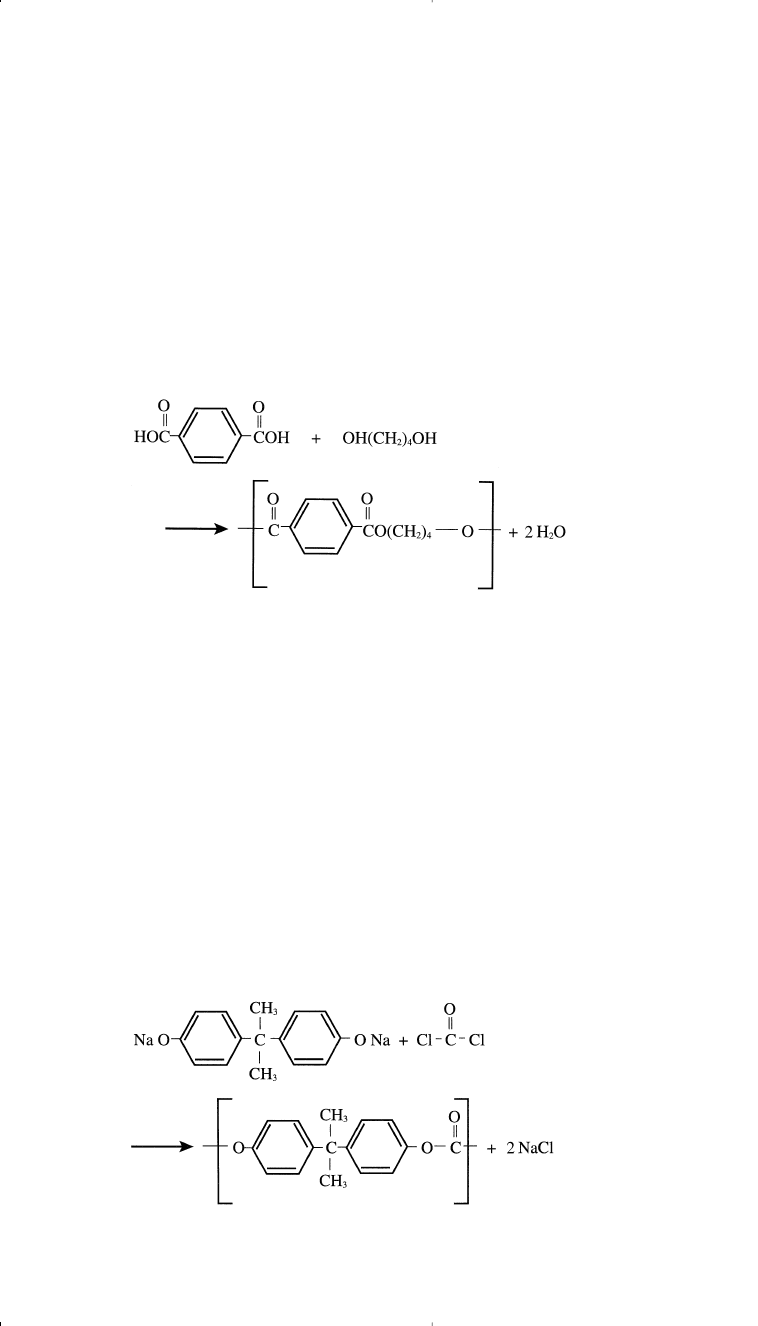
POLYCARBONATES
Polycarbonates (PC) are another group of condensation thermoplastics
used mainly for special engineering purposes. These polymers are con-
sidered polyesters of carbonic acid. They are produced by the condensa-
tion of the sodium salt of bisphenol A with phosgene in the presence of
an organic solvent. Sodium chloride is precipitated, and the solvent is
removed by distillation:
Chapter 12 1/22/01 11:11 AM Page 337
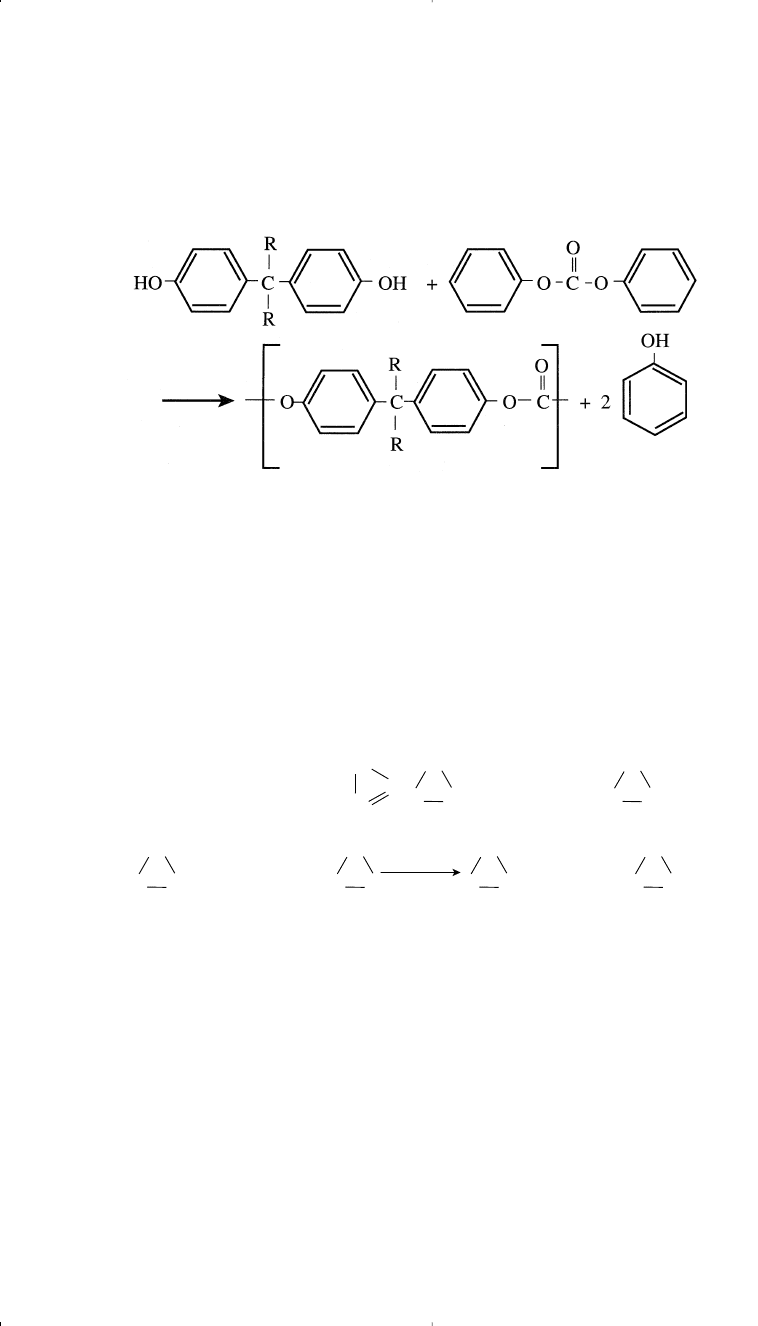
Another method for producing polycarbonates is by an exchange reaction
between bisphenol A or a similar bisphenol with diphenyl carbonate:
338 Chemistry of Petrochemical Processes
Diphenol carbonate is produced by the reaction of phosgene and phe-
nol. A new approach to diphenol carbonate and non-phosgene route is by
the reaction of CO and methyl nitrite using Pd/alumina. Dimethyl car-
bonate is formed which is further reacted with phenol in presence of
tetraphenox titanium catalyst. Decarbonylation in the liquid phase yields
diphenyl carbonate.
However, the reaction is equilibrium constained and requires a compli-
cated processing scheme.
24
Properties and Uses of Polycarbonates
Polycarbonates, known for their toughness in molded parts, typify the
class of polymers known as engineering thermoplastics. These materials,
designed to replace metals and glass in applications demanding strength
and temperature resistance, offer advantages of light weight, low cost,
and ease of fabrication.
25
Materials made from polycarbonates are transparent, strong, and heat-
and break-resistant. However, these materials are subject to stress crack-
OO
|| ||
CO + 2 CH
3
ONO r CH
3
O—C—COCH
3
+ 2NO
OO O
|| || ||
—O—C—C—O— —O—C—O— + CO
@
@
Decarbon.
@
@
OO OO
|| || || ||
CH
3
O—C—C—OCH
3
+ 2
@
r —O—C—C—O— + 2 CH
3
OH
@
@
Chapter 12 1/22/01 11:11 AM Page 338

ing and can be attacked by weak alkalies and acids. Table 12-4 compares
the properties of polycarbonates with other thermoplastic resins.
25
Polycarbonates are used in a variety of articles such as laboratory
safety shields, street light globes, and safety helmets. The maximum
usage temperature for polycarbonate objects is 125°C.
POLYETHER SULFONES
Polyether sulfones (PES) are another class of engineering thermoplas-
tics generally used for objects that require continuous use of tempera-
tures around 200°C. They can also be used at low temperatures with no
change in their physical properties.
Synthetic Petroleum-Based Polymers 339
Table 12-4
Properties of polycarbonates compared
with some thermoplastics
25
Melting or
glass Izod
transition tensile compressive flexural impact,
temperature strength strength strength 1/8 in.
Resin (°C) (MPa) (MPa) (MPa) (J/m)
PPO, impact 100–110 117–127 124–162 179–200 43–53
modified
PC 149 65 86 93 850
PC, 30% glass 149 131 124 158 106
PC-ABS 149 48–50 76 89–94 560
Nylon 6/6, impact 240–260 48–55 160–210
modified
Nylon 6/6, 33% 265 151–193 202 282 117–138
glass
PBT 232–267 56 59–100 82–115 43–53
PBT, 30% glass 232–267 117–131 124–162 179–200 69–85
Acetal, 181 124 96 69–122
homopolymer
ABS, impact 100–110 33–43 31–55 55–76 347–400
modified
PPO, impact 135 48–55 69 56–76 320–370
modified
PPO, 30% 100–110 117–127 123 138–158 90–112
reinforced
Chapter 12 1/22/01 11:11 AM Page 339
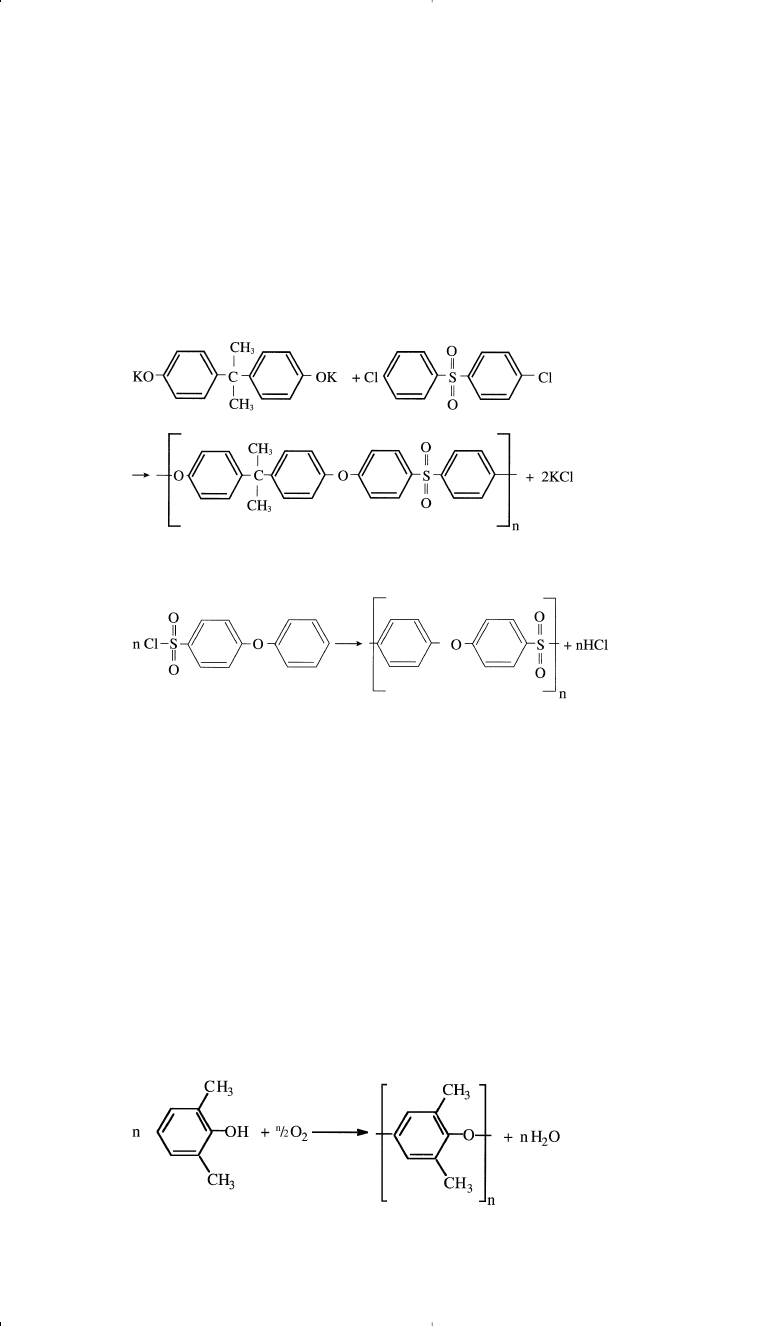
Polyether sulfones can be prepared by the reaction of the sodium or
potassium salt of bisphenol A and 4,4
v
-dichlorodiphenyl sulfone.
Bisphenol A acts as a nucleophile in the presence of the deactivated aro-
matic ring of the dichlorophenylsulfone. The reaction may also be cat-
alyzed with Friedel-Crafts catalysts; the dichlorophenyl sulfone acts as
an electrophile:
340 Chemistry of Petrochemical Processes
Polyether sulfones could also be prepared using one monomer:
Properties and Uses of Aromatic Polyether Sulfones
In general, properties of polyether sulfones are similar to those of
polycarbonates, but they can be used at higher temperatures. Figure 12-6
shows the maximum use temperature for several thermoplastics.
26
Aromatic polyether sulfones can be extruded into thin films and foil and
injection molded into various objects that need high-temperature stability.
POLY(PHENYLENE) OXIDE
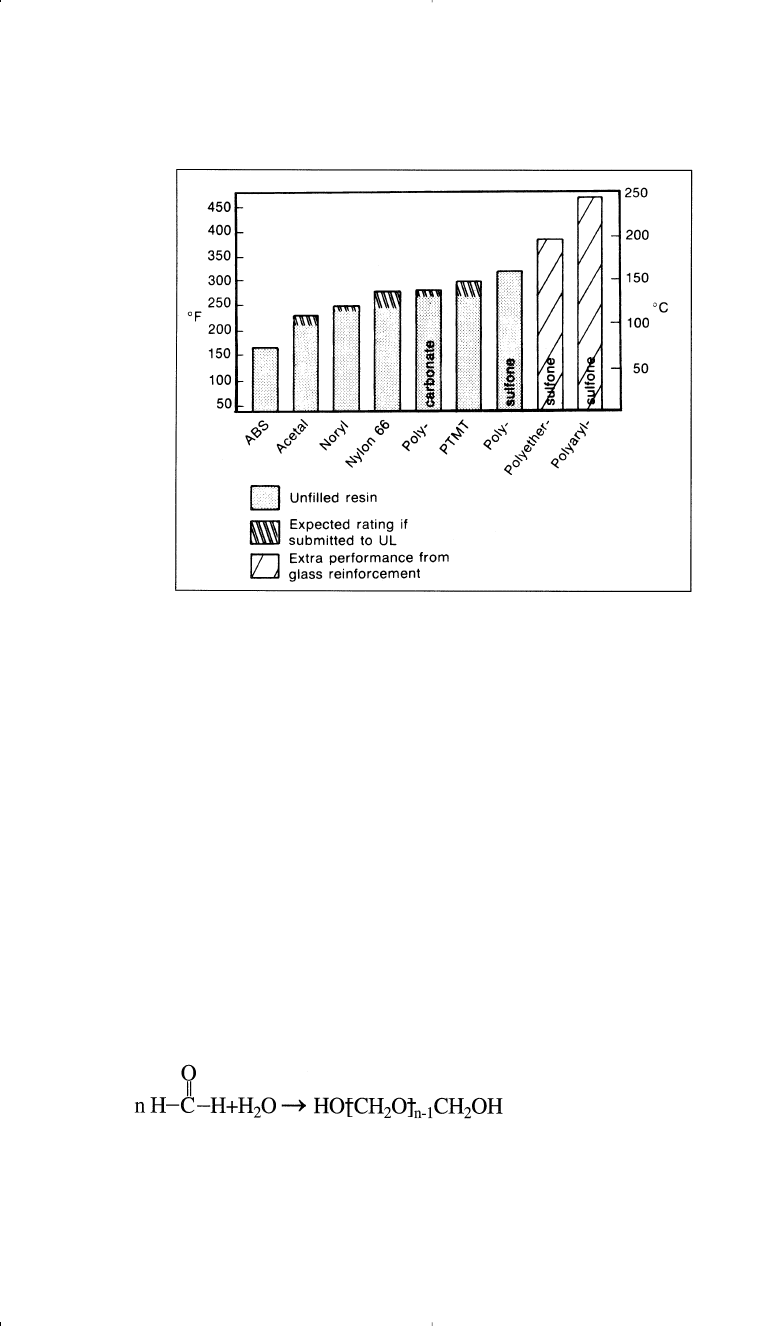
Polyphenylene oxide (PPO) is produced by the condensation of 2,6-
dimethylphenol. The reaction occurs by passing oxygen in the phenol
solution in presence of Cu
2
Cl
2
and pyridine:
Chapter 12 1/22/01 11:11 AM Page 340
PPO is an engineering thermoplastic with excellent properties. To
improve its mechanical properties and dimensional stability, PPO can be
blended with polystyrene and glass fiber. Articles made from PPO could
be used up to 330°C: it is mainly used in items that require higher tem-
peratures such as laboratory equipment, valves, and fittings.
POLYACETALS
Polyacetals are among the aliphatic polyether family and are produced
by the polymerization of formaldehyde. They are termed polyacetals to
distinguish them from polyethers produced by polymerizing ethylene
oxide, which has two -CH
2
- groups between the ether group. The poly-
merization reaction occurs in the presence of a Lewis acid and a small
amount of water at room temperature. It could also be catalyzed with amines:
Synthetic Petroleum-Based Polymers 341
Figure 12-6. Maximum continuous use temperature of some engineering thermo-
plastics.
26
Polyacetals are highly crystalline polymers. The number of repeating
units ranges from 500 to 3,000. They are characterized by high impact
resistance, strength, and a low friction coefficient.
Chapter 12 1/22/01 11:11 AM Page 341
Articles made from polyacetals vary from door handles to gears and
bushings, carburetor parts to aerosol containers. The major use of poly-
acetals is for molded grades.
THERMOSETTING PLASTICS
This group includes many plastics produced by condensation polymer-
ization. Among the important thermosets are the polyurethanes, epoxy
resins, phenolic resins, and urea and melamine formaldehyde resins.
POLYURETHANES
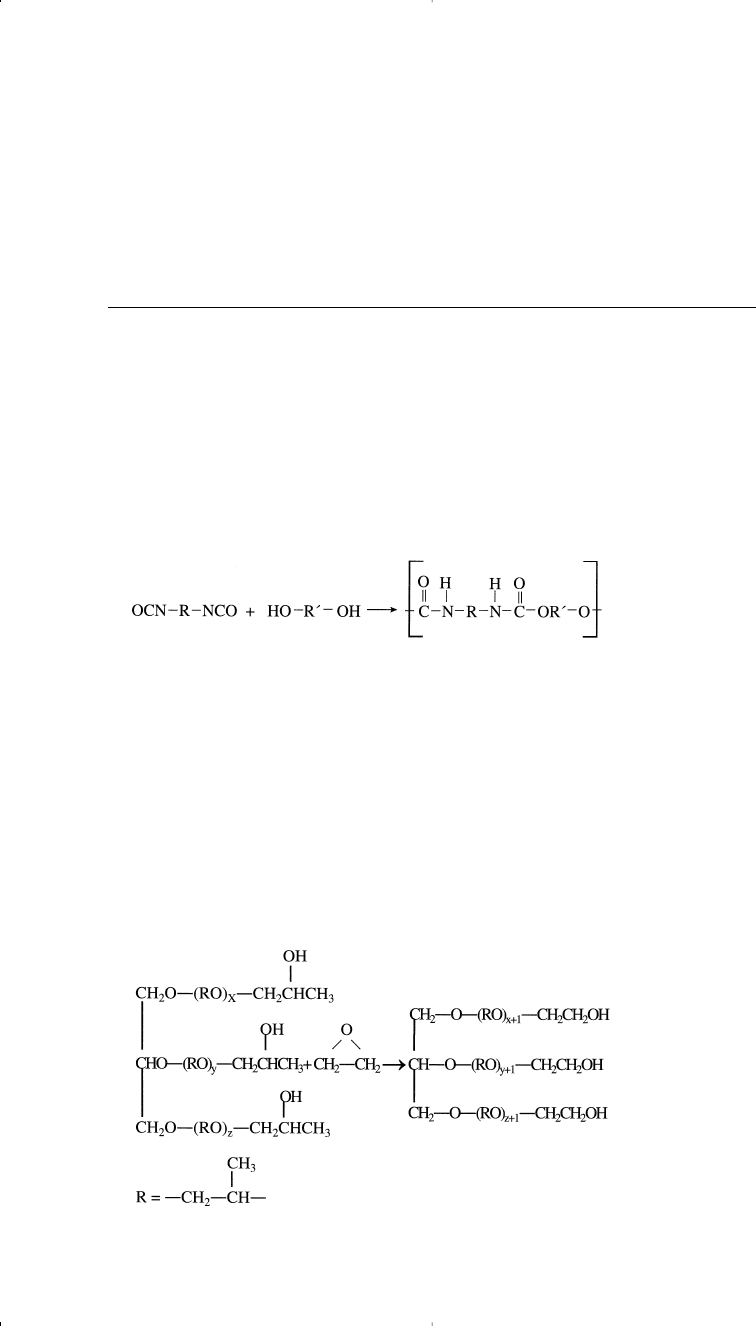
Polyurethanes are produced by the condensation reaction of a polyol
and a diisocyanate:
342 Chemistry of Petrochemical Processes
No by-product is formed from this reaction. Toluene diisocyanate
(Chapter 10) is a widely used monomer. Diols and triols produced from
the reaction of glycerol and ethylene oxide or propylene oxide are suit-
able for producing polyurethanes.
Polyurethane polymers are either rigid or flexible, depending on the
type of the polyol used. For example, triols derived from glycerol and
propylene oxide are used for producing block slab foams. These polyols
have moderate reactivity because the hydroxy groups are predominantly
secondary. More reactive polyols (used to produce molding polyurethane
foams) are formed by the reaction of polyglycols with ethylene oxide to
give the more reactive primary group:
27
Chapter 12 1/22/01 11:11 AM Page 342

Other polyhydric compounds with higher functionality than glycerol
(three-OH) are commonly used. Examples are sorbitol (six-OH) and
sucrose (eight-OH). Triethanolamine, with three OH groups, is also used.
Diisocyanates generally employed with polyols to produce polyure-
thanes are 2,4-and 2,6-toluene diisocyanates prepared from dinitro-
toluenes (Chapter 10):
Synthetic Petroleum-Based Polymers 343
A different diisocyanate used in polyurethane synthesis is methylene
diisocyanate (MDI), which is prepared from aniline and formaldehyde.
The diamine product is reacted with phosgene to get MDI.
The physical properties of polyurethanes vary with the ratio of the
polyol to the diisocyanate. For example, tensile strength can be adjusted
within a range of 1,200–600 psi; elongation, between 150–800%.
28
Improved polyurethane can be produced by copolymerization. Block
copolymers of polyurethanes connected with segments of isobutylenes
exhibit high-temperature properties, hydrolytic stability, and barrier char-
acteristics. The hard segments of polyurethane block polymers consist of
(–RNHCOO)–
n
, where R usually contains an aromatic moiety.
29
Properties and Uses of Polyurethanes
The major use of polyurethanes is to produce foam. The density as
well as the mechanical strength of the rigid and the flexible types varies
widely with polyol type and reaction conditions. For example,
polyurethanes could have densities ranging between 1–6 lb/ft
3
for the
flexible types and 1–50 lb/ft
3
for the rigid types. Polyurethane foams
have good abrasion resistance, low thermal conductance and good load-
bearing characteristics. However, they have moderate resistance to
organic solvents and are attacked by strong acids. Flame retardency of
polyurethanes could be improved by using special additives, spraying a
coating material such as magnesium oxychloride, or by grafting a halo-
gen phosphorous moiety to the polyol. Trichlorobutylene oxide is
Chapter 12 1/22/01 11:11 AM Page 343
sometimes copolymerized with ethylene and propylene oxides to pro-
duce the polyol.
Major markets for polyurethanes are furniture, transportation, and
building and construction. Other uses include carpet underlay, textural
laminates and coatings, footwear, packaging, toys, and fibers.
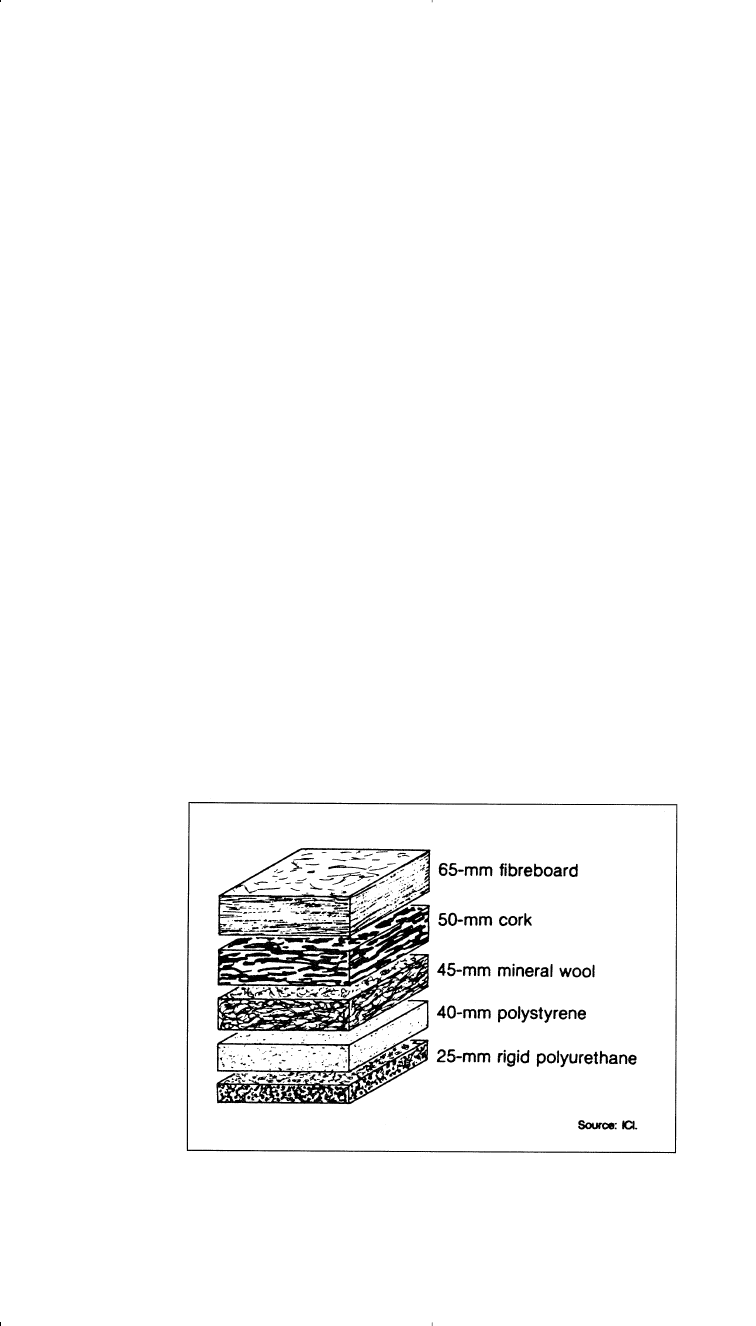
The largest use for rigid polyurethane is in construction and industrial
insulation due to its high insulating property. Figure 12-7 compares the
degree of insulation of some insulating materials.
28
Molded urethanes are used in items such as bumpers, steering wheels,
instrument panels, and body panels. Elastomers from polyurethanes are
characterized by toughness and resistance to oils, oxidation, and abra-
sion. They are produced using short-chain polyols such as polytetram-
ethylene glycol from 1,4-butanediol. Polyurethanes are also used to
produce fibers. Spandex (trade name) is a copolymer of polyurethane
(85%) and polyesters.
Polyurethane networks based on triisocyante and diisocyanate connected
by segments consisting of polyisobutylene are rubbery and exhibit high
temperature properties, hydrolyic stability, and barrier characteristics.
29
EPOXY RESINS
Epoxy resins are produced by reacting epichlorohydrin and a diphe-
nol. Bisphenol A is the diphenol generally used. The reaction, a ring
344 Chemistry of Petrochemical Processes
Figure 12-7. The comparative thickness for the same degree of insulation (dry
conditions).
28
Chapter 12 1/22/01 11:11 AM Page 344
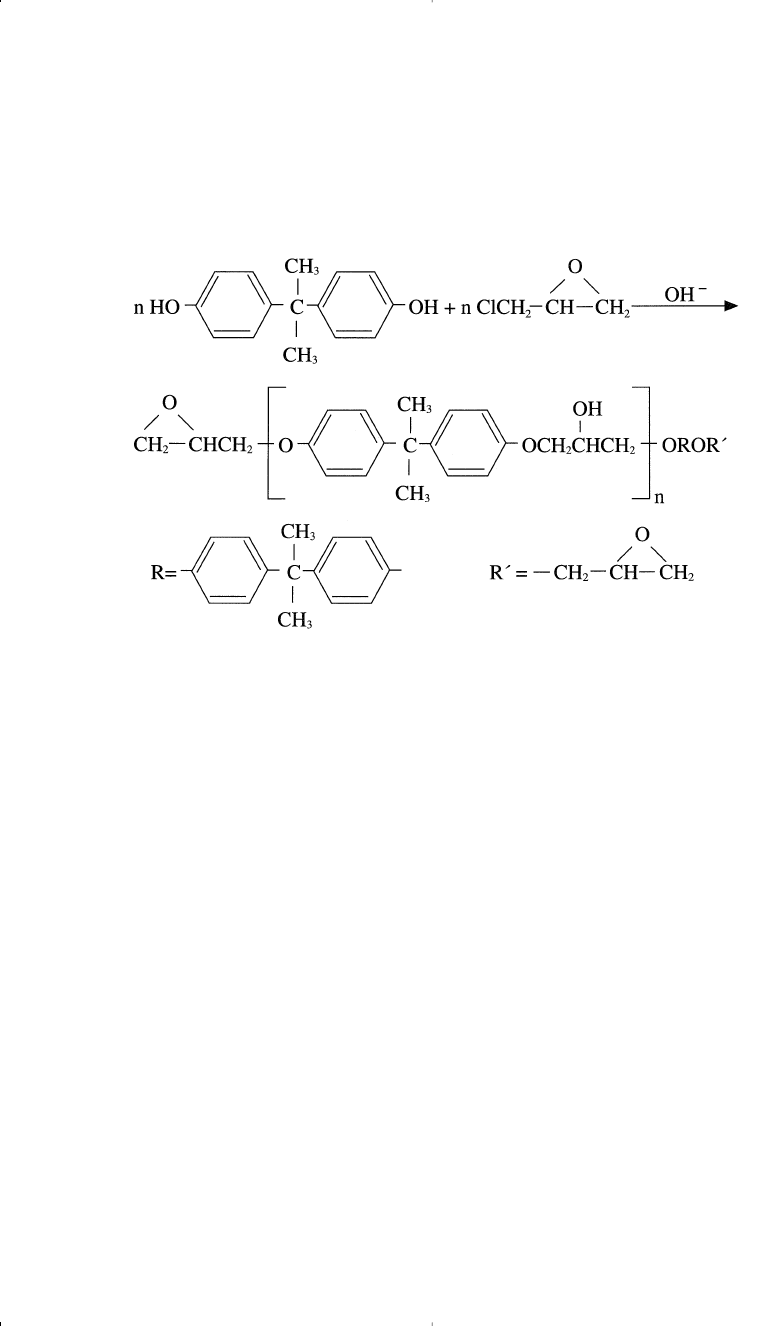
opening polymerization of the epoxide ring, is catalyzed with strong
bases such as sodium hydroxide. A nucleophilic attack of the phenoxy
ion displaces a chloride ion and opens the ring:
Synthetic Petroleum-Based Polymers 345
The linear polymer formed is cured by cross-linking either with an
acid anhydride, which reacts with the -OH groups, or by an amine, which
opens the terminal epoxide rings. Cresols and other bisphenols are also
used for producing epoxy resins.
Properties and Uses of Epoxy Resins
Epoxy resins have a wide range of molecular weights (≈1,000–10,000).
Those with higher molecular weights, termed phenoxy, are hydrolyzed
to transparent resins that do not have the epoxide groups. These could
be used in molding purposes, or crosslinked by diisocyanates or by
cyclic anhydrides.
Important properties of epoxy resins include their ability to adhere
strongly to metal surfaces, their resistance to chemicals, and their high
dimensional stability. They can also withstand temperatures up to 500°C.
Epoxy resins with improved stress cracking properties can be made by
using toughening agents, such as carboxyl-terminated butadiene-acry-
lonitrile liquid polymers. The carboxyl group reacts with the terminal
epoxy ring to form an ester. The ester, with its pendant hydroxyl groups,
reacts with the remaining epoxide rings, then more crosslinking occurs
by forming ether linkages. This material is tougher than epoxy resins and
suitable for encapsulating electrical units.
Chapter 12 1/22/01 11:11 AM Page 345
Major uses of epoxy resins are coatings for appliance finishes, auto
primers, adhesive, and in coatings for cans and drums. Interior coatings of
drums used for chemicals and solvents manifests its chemical resistance.
In 1997, approximately 681 million pounds of unmodified epoxy
resins were produced in the U.S.
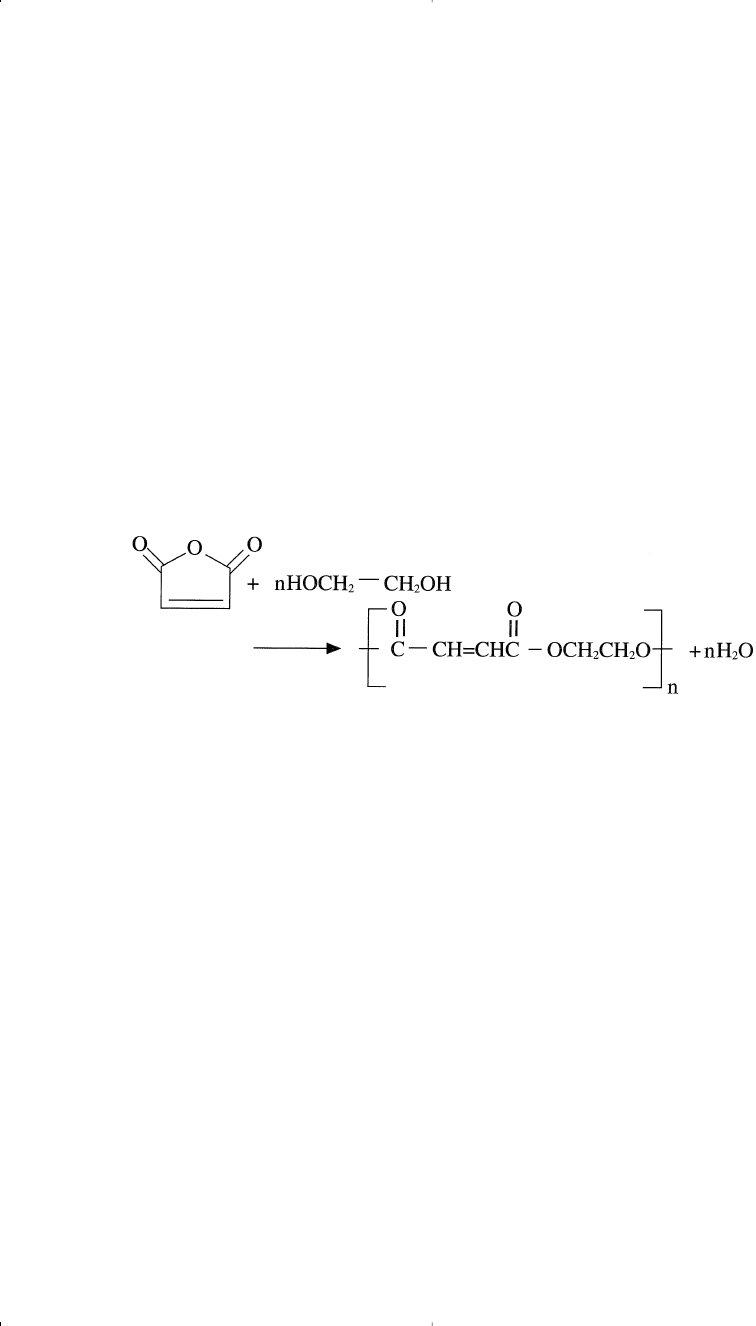
UNSATURATED POLYESTERS
Unsaturated polyesters are a group of polymers and resins used in
coatings or for castings with styrene. These polymers normally have
maleic anhydride moiety or an unsaturated fatty acid to impart the
required unsaturation. A typical example is the reaction between maleic
anhydride and ethylene glycol:
346 Chemistry of Petrochemical Processes
Phthalic anhydride, a polyol, and an unsaturated fatty acid are usually
copolymerized to unsaturated polyesters for coating purposes. Many
other combinations in variable ratios are possible for preparing these
resins. The 1998 U.S. production of polyesters was approximately 1.7
billion pounds.
PHENOL-FORMALDEHYDE RESINS
Phenol-formaldehyde resins are the oldest thermosetting polymers.
They are produced by a condensation reaction between phenol and
formaldehyde. Although many attempts were made to use the product and
control the conditions for the acid-catalyzed reaction described by Bayer
in 1872, there was no commercial production of the resin until the exhaus-
tive work by Baekeland was published in 1909. In this paper, he deseribes
the product as far superior to amber for pipe stem and similar articles, less
flexible but more durable than celluloid, odorless, and fire-resistant.
30
The reaction between phenol and formaldehyde is either base or acid
catalyzed, and the polymers are termed resols (for the base catalyzed)
and novalacs (for the acid catalyzed).
Chapter 12 1/22/01 11:11 AM Page 346
