Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.

516 Urena, Mendez-Torres, and Thomas
Most steinstrasse are short, transient, and asymptomatic, or are passed causing just
mild discomfort. However, larger steinstrasse can produce partial or complete ureteral
obstruction and become symptomatic and/or cause sepsis. In this latter case, its early
recognition and prompt management avoid escalation of symptoms and risk of urosep-
sis. Anuria may complicate steinstrasse in case of an obstructed solitary kidney or after
bilateral SWL. In almost a third of patients, obstruction may be silent (46), leading to
ipsilateral renal function deterioration. In the absence of symptoms, silent obstruction
can be ruled out with follow up renal US or intravenous pyelogram (IVP).
A decision to place a ureteral stent should be made after all the risk factors for
steinstrasse development have been taken into consideration. Pre-SWL ureteral stents
initially allow for the passage of urine and gravel through and around it, respectively. It
is believed that the urine that refluxes from the bladder back to the kidney through the
ureteral stent is responsible for contributing to ureteral peristalsis, which then aids to
propel urine and gravel down to the bladder (47). With time, the ureteral stent also
produces passive ureteral dilation (48,49), and this allows for passage of larger frag-
ments.
Routine use of ureteral stents is not advocated because its use is not without associated
potential morbidity. It has been found to produce discomfort and intolerance that may
require its removal and even to have caused obstruction by itself (50). Besides this, it
increases the level of invasiveness and cost associated with delivery of SWL (51).
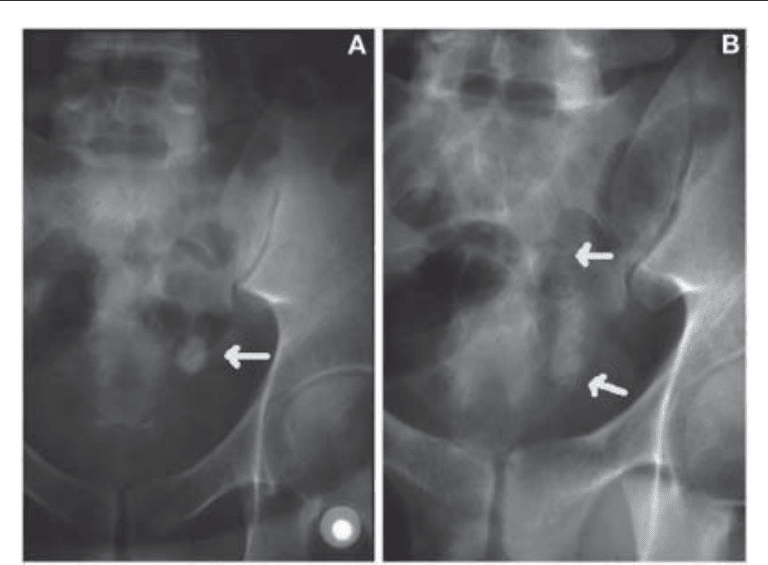
Fig. 2. (A) Large ureteral stone (arrow). (B) Same ureteral stone after SWL showing steinstrasse
(between arrows) and renal obstruction.

Chapter 27 / Complications of Urinary Stone Surgery 517
Much debate has existed with regard to proper pre-SWL stenting based on stone size.
Low et al. (52) found that placement of double J stents for improving stone-free rates,
alleviating pain, or preventing ureteral obstruction in conjunction with SWL of solitary
renal calculi <20 mm in diameter was unnecessary. In a study of 1087 patients, with
stones ranging in size from 10 to 95 mm, Sulaiman et al. (43) reported that the use of
ureteral stents had no effect on the incidence of steinstrasse for patients with lithiasis <2
cm, but they did find a much lower likelihood of steinstrasse and steinstrasse-associated
symptoms in patients with lithiasis >2 cm in whom a stent was placed before SWL.
Similarly, Al-Awadi et al. (53) found that the use of pre-SWL stents significantly low-
ered the incidence of steinstrasse in patients with a stone burden of 1.5–3.5 cm, and that
the incidence of steinstrasse increased with the size of the calculi, whether a stent was
placed before SWL or not. Despite the lack of evidence to support the use of stents in
uncomplicated cases with smaller stones, a survey done by the American Urological
Association revealed that one-fourth of the respondents use pre-SWL stent for renal
pelvic stones up to 1 cm in size, and that more than half routinely stent patients with
renal calculi of 1.5 cm (54).
Patient selection is the key for steinstrasse prevention. The risks, benefits, and costs
of pre-SWL ureteral stenting should be weighed. Although ureteral stents may not pre-
vent steinstrasse nor improve stone-free rates in patients with larger stones, they prevent
obstruction from steinstrasse and are recommended in patients with stone burden >2 cm
(43,44,51). Similarly, although associated with more irritative symptoms, pre-SWL
ureteral stent placement is recommended when treating solitary kidneys with lithiasis
10–20 mm or solitary proximal ureteral stones <2 cm because they are associated with
fewer hospital readmissions and emergency room visits (55), besides assisting in stone
localization.
The management of steinstrasse depends on the clinical situation. Asymptomatic or
minimally symptomatic steinstrasse can often be managed conservatively as long as
there is no risk of jeopardizing the renal function; in these cases, spontaneous stone
clearance has been found to occur in 60–86% (44,46,51,56). The treatment options for
symptomatic steinstrasse vary according to its location in the ureter, size, grade of
ureteral obstruction, and presence of urinary infection. They include percutaneous or
endoscopic retrograde drainage and SWL. Steinstrasse should be treated in all cases
when: conservative management fails, pain is refractory to medical treatment, there is
distal unilateral or bilateral obstruction, obstruction in a solitary kidney, and/or urosep-
sis. Under any of these circumstances, prompt urinary tract decompression should be
considered with either a retrograde stent placement or a percutaneous nephrostomy.
Most short steinstrasse clear without requiring any intervention, as reported by Kim
et al. (56), with 64% of their patients having steinstrasse of an average length of 2.6 cm,
and being treated conservatively. The use of prophylactic antibiotics is recommended
when conservative management is chosen, as well as are frequent radiologic and ultra-
sound examinations. Failure of steinstrasse to resolve with expectant management within
3–4 wk may necessitate intervention (58). Whenever intervention is required, the least
invasive and more effective procedure is advocated.
In situ SWL, in the absence of a distal obstruction and/or sepsis, can be used either for
steinstrasse in the upper and lower ureter or for a leading ureteral calculus fragment.
Success rates of up to 90% with minimal complications have been reported for repeated
SWL (43,56). In situ repeat SWL either disintegrates the lead fragment or mechanically
loosens it (44). Ureteroscopy with intracorporeal lithotripsy alone and/or with stone

518 Urena, Mendez-Torres, and Thomas
basket extraction are often used in uncomplicated cases of simple steinstrasse (column
of gravel <5 cm and without urosepsis) (59) or in cases of a large ureteral leading
fragment, whenever a guidewire can be passed beyond the obstruction. Even though
success rates of almost 100% have been achieved with ureteroscopy (43,58,60), caution
is warranted because of a higher risk of ureteral perforation in these cases (53). For
steinstrasse in the distal ureter, either SWL or ureteroscopy could be attempted.
Complex steinstrasse with associated sepsis, or the inability to pass a guidewire
through the site of obstruction, is an indication for percutaneous drainage. Percutaneous
nephrostomy drainage alone, besides relieving obstruction and/or infection, decreases
the intrapelvic pressure, which re-establishes ureteric peristalsis and thus permits the
passage of stone fragments in up to 75% of cases (44,58). PCNL can also be used for
steinstrasse with or without ureterorenoscopy. In case of a combined approach, an
antegrade guidewire can be passed through the nephrostomy tract and aid in the retro-
grade ureteroscopic approach. Ureteral meatomy (61) to facilitate fragment passage and
retrograde steinstrasse irrigation (62) have been reported, but are rarely used today.
In summary, given the high likelihood of associated morbidity, if a patient has a high
probability of steinstrasse formation, close follow-up with early intervention or prophy-
lactic pre-SWL ureteral stenting should be considered (45).
Infection
Many stones may harbor bacteria even though bacteriuria is only intermittently
present, especially in patients who have been treated previously with antibiotics. It has
been documented that in 5–16% of patients undergoing SWL, bacteriuria will develop
despite a sterile urine culture before lithotripsy (63). However, only 2–3% of these
patients develop a symptomatic urinary tract infection (UTI) (63). No significant corre-
lation was found between the occurrence of bacteriuria and the number and size of the
stones, nor was there any correlation between bacteriuria and the stone-free rate or the
location of the calculi after SWL therapy (64). There is evidence that urine cultures may
not be a reliable indicator of bacteria within stones, making it difficult to predict all
patients in whom infectious complications might develop after SWL.
Fragmentation of infectious stones, despite sterile urine, may release preformed bac-
terial endotoxins and viable bacteria into the surrounding medium that may be later
absorbed systemically. The renal trauma and microvasculature disruption associated
with SWL allow bacteria to enter the bloodstream. Although bacteremia has been
reported in up to 14% after SWL (65), the overall reported incidence of sepsis related to
SWL therapy is less than 1% and 2.7% for nonstaghorn calculi and staghorn calculi,
respectively (66). The risk of sepsis increases if the urine culture is positive before SWL
and especially in the presence of obstruction (67). Therefore, bacteriologic evaluation
of the urine is recommended in these patients and SWL should be performed only in
cases in which there is no evidence of urinary tract infection and distal obstruction.
Controversy exists regarding the routine use of prophylactic antibiotics in SWL.
Dincel et al. (64) found that there was a significantly higher risk of UTI in patients with
struvite stones than in those with other types of stones (17.3% vs 2.1%). They concluded
that although prophylactic antimicrobial therapy was justifiable among patients with a
history of UTI or a suspected struvite stone, even in the absence of bacteriuria, it was of
little value in patients with a calcium oxalate or calcium phosphate stone. Bierkens et al.
(68), in a prospective, placebo-controlled randomized study corroborated these findings.
They noted that in a group of patients with documented sterile urine who received

Chapter 27 / Complications of Urinary Stone Surgery 519
prophylactic antibiotics immediately before and after SWL, and a controlled group, the
incidence of pyuria or bacteriuria did not change and that in both groups only 2–3% of
the patients eventually had clinical and bacteriological signs of a UTI within 6 wk of
SWL. Similarly, Clayman and colleagues (63)explained that their SWL treatment policy
had changed and that only patients with a suspected struvite stone or those undergoing
ureteral stent placements before lithotripsy received prophylactic antibiotics.
On the other hand, in a meta-analysis of multiple randomized studies, Pearle and
Roehrborn(69) concluded that antibiotic prophylaxis before SWL in patients with sterile
pretreatment urine cultures was efficacious in reducing the rate of post-SWL UTIs, and
that it was both efficacious and cost-effective in all patients undergoing SWL when the
need for inpatient treatment of urosepsis or pyelonephritis was taken into consideration.
Preoperative antibiotics should thus be administered to patients who have radiographic
or clinical features suggestive of infectious stones (i.e., struvite, staghorn calculus), in
whom infection is highly suspected or with a positive urine culture, in patients with
recurrent UTI, and in those undergoing any kind of endoscopic instrumentation associ-
ated with SWL.
Although less common, other infectious complications of SWL have been described.
They include perinephric and psoas abscess, miliary tuberculosis, endocarditis, fungal
and bacterial endophthalmitis, and death (70–77). Knowledge of the potential existence
of these infectious complications, coupled with early recognition and proper manage-
ment, will minimize posttreatment morbidity.
Complications Secondary to Effects of SWL on Tissue:
Renal Complications
BLEEDING
A variety of renal injuries can occur after SWL. Among these, the most common
presenting sign is gross hematuria. Hematuria is commonly seen without clinical signifi-
cance and usually resolves within the first 12 h (2,78). It has been observed in patients
receiving more than 2000 shocks (2,78), and to occur regardless of the type of lithotriptor
used. Although initially believed to be urothelial injury caused by stone fragments, it is
now known to be the direct result from shockwave induced renal injury. Animal models
without renal calculi produced parenchymal injury and hematuria following shockwave
lithotripsy with either HM-3 or a piezoelectric lithotriptor (79,80), as did patients who
underwent biliary lithotripsy (81). These studies confirm that hematuria resulted sec-
ondary to the renal injury from the shockwaves and not from the shock effect on the
calculus.
Shockwave-induced renal trauma has been studied and identified radiographically,
histopathologically, and by means of biochemical and isotope markers. In 63–85% of
patients treated with SWL, renal injuries were noted with US, CT, MRI or quantitative
radionuclide renography studies (82–85). Radiographically, the renal lesions more fre-
quently noted include perirenal hematoma, renal enlargement, renal fracture, loss of
corticomedullary junction demarcation, and low signal intensity changes in perirenal fat
(86). Among these, hemorrhage and edema within or around the kidney are the most
often seen. Diffuse or focal kidney enlargement and loss of corticomedullary junction
are present in cases of intrarenal edema. MRI and CT scans have been proven to be more
sensitive than ultrasound in detecting and monitoring these renal structural changes after
SWL treatment (84,87).

520 Urena, Mendez-Torres, and Thomas
The urinary elevation of several enzymes and proteins has been used as biochemical
markers to document shockwave injury to nephrons and the surrounding soft tissues.
The elevated biochemical markers include proximal tubular enzymes, such as N-acetyl-
β-
D-glucosaminidase and β-galactosidase, renal brush border epithelial cells enzymes,
γ-glutamyltransaminase and angiotensin converting enzyme and renal tubular enzymes
β-microglobulin, as well as urothelial glycosaminoglycan and glucose, proteins, and
immunoglobulin G alterations in cases of diffuse renal trauma (88–101). Although
most studies have found significant elevated levels of these biochemical markers,
Krongrad et al. (102) did not find a statistically significant increase of these tubular
enzymes, suggesting that their elevation was a result of a tubular defect in association
with stone disease and not a response to SWL treatment. Other less specific markers
have also been found to be transiently elevated after SWL, but are not just associated
with shockwave-related injury to the kidney but also to adjacent organs and soft tissues.
These include alkaline phosphatase, lactic dehydrogenase, glutamic-pyruvic transami-
nase, glutamic-oxaloacetic transaminase, C-reactive protein, S-100 protein, and crea-
tinine kinase.
Histopathologic studies in animals and humans have also documented acute changes
in the kidneys and surrounding tissues after SWL. Tubular, vascular, and interstitial
changes have been localized to the plane of the pressure wave, in which disruption of
the renal parenchymal cells have been found, as well as degenerative changes and
accumulation of hemosiderin granules and cast material (103). Dilation of veins with
evidence of endothelial damage and thrombus formation was among the alterations
found within the microvasculature (103). The effect of increasing the number of
shockwaves is translated into a higher rate of damage to the nephrons and especially to
small- and medium-sized blood vessels within the F2 range. This explains why although
electrohydraulic lithotriptors produce a larger lesion, electromagnetic lithotriptors,
because of more cellular destruction at F2, are associated with a higher rate of subcap-
sular hemorrhage.
Retroperitoneal hematomas have been documented radiographically in intraparen-
chymal, subcapsular, and perirenal locations. These hematomas have been found to be
produced primarily as a result of shockwave-induced vascular insult to thin-walled
veins and walls of small arteries and glomerular and peritubular capillaries. Rupture
of these interlobular and arcuate veins located in the corticomedullary junction makes
this region of the kidney more vulnerable to SWL-related intrarenal hematoma and
hemorrhage. Histopathology analysis after SWL has shown that although glomerular
atrophy and sclerosis, as well as interstitial fibrosis and hyalinization of arcuate veins,
are present, the rest of the renal parenchyma appears normal (89,104), suggesting that
SWL-related renal injury is focal and that this does not affect the majority of the renal
parenchyma.
Perirenal and subcapsular fluid collections, either from bleeding or urine, occur in 24–
32% of patients after SWL (78,83,84). Their appearances vary from mild intraparenchy-
mal contusions to large hematomas. Most hematomas are usually asymptomatic, with
symptomatic hematomas being reported in less than 1% of patients (105,106). These
hematomas are more commonly found in hypertensive patients, especially those with
poor blood pressure control at the time of treatment, and among patients on antiplatelet
medication (84,107,108). Other risk factors for increased hemorrhage and also related
to vascular disorders, are advanced arteriosclerosis, primary coagulopathic disorders,
diabetes mellitus, coronary artery disease, and obesity (109,110). No correlation in the

Chapter 27 / Complications of Urinary Stone Surgery 521
occurrence of hematomas was found when the voltage applied and number of shockwaves
administered were evaluated (109,110).
Although patients with uncorrected coagulopathies and thrombocytopenia have higher
risk of hematoma formation, SWL has been successfully performed among both hemo-
philiac patients and patients with bleeding diathesis after adequate preoperative work up
and corrective measures (111,112). Most renal hematomas are managed conservatively.
Perirenal fluid collection reabsorbs within a few days, whereas subcapsular hematomas
may take from 6 wk to 6 mo to resolve radiographically (88,113,114). Surgical manage-
ment may be warranted in cases of persisting symptomatic renal hematomas or progres-
sive renal function impairment (106,115). Rarely, large hematomas may need blood
transfusions or may produce a state of acute renal failure, which may result in death if
the condition is not recognized and treated promptly.
R
ENAL FUNCTION POST-SHOCKWAVE LITHOTRIPSY
Ever since the early studies on the use of SWL were reported, efforts have been
made to address its effects on renal function. To date, it has not been convincingly
proven whether renal function is altered in all patients undergoing SWL, or if only in
a subset of patients who are at risk. It is not clearly understood whether patients with
two kidneys tolerate SWL therapy better than patients with just one kidney. Although
acute renal failure has been described, the condition can be reversible (116) or asso-
ciated with renal loss owing to either the presence of perirenal hematomas or silent
obstruction (117).
Extensive animal studies have been performed to evaluate the effects of SWL on
glomerular filtration rate (GFR) and renal plasma flow (RPF). Willis et al. (118) first
studied the effects of SWL on renal hemodynamics in anesthetized minipigs with and
without pretreatment with verapamil. They found a significantly acute reduction in GFR
and RPF of the shocked kidneys, as well as a significant reduction of the RPF of the
contralateral unshocked kidney, but not of its GFR. Verapamil was found to blunt the
SWL-induced reductions of urine flow, GFR, and RPF in the shocked kidneys and to
eliminate the reduction of RPF in the unshocked kidneys. In another study using unine-
phrectomized and binephric minipigs, Willis et al. (119)found that although a reduction
in GFR and RPF after SWL occurred in both groups, no differences were found in
whole-animal GFR and RPF in either group before or after SWL. They attributed this
to the compensatory renal hypertrophy and improved hemodynamics in solitary kid-
neys, which may acutely attenuate the renal vasoconstrictive effect of SWL (119).
Similar results have been achieved after studying the effects of SWL on renal
function in humans. Kaude et al. (78)
and Thomas et al. (85) found an immediate
decrease in effective renal plasma flow, measured by renal scans, in 30% of kidneys
treated with SWL. The decrease in renal function has been related to the number of
shocks received by the shocked nephron units at F2, where a transient reduction of
intrarenal blood flow was observed (120–122). These studies indicate that renal func-
tion is adversely affected acutely in some patients after SWL and that the primary
change appears to be a vasoconstrictive response resulting in a decrease in both GFR
and RPF.
B
ILATERAL SHOCKWAVE LITHOTRIPSY
Long-term studies of the role of SWL on renal function show variable results. Some
studies have reported a reduction on GFR or an increase of serum creatinine whereas

522 Urena, Mendez-Torres, and Thomas
others found no difference. Five years after shockwave lithotripsy, Brito et al. (123)
noted a 40% rise in serum creatinine and a 10% fall in the estimated RPF rate in seven
patients. Cass (20) reported a significant decrease in GFR of >20% in 18% and 13% of
patients after simultaneous bilateral SWL and after SWL to solitary kidneys, respec-
tively. Thomas et al. (85) reported a statistically significant decrease in renal function
RPF with synchronous bilateral SWL treatment. At 4.5 yr after shockwave lithotripsy in
patients with a solitary kidney, Chandhoke et al. (124) also noted a decrease of 22% in
the GFR, which was similar to the 29% long-term reduction in renal function recorded
after percutaneous nephrolithotomy in solitary kidneys. SWL-induced renal trauma
results in a potential focal scarring and a mild decrease in renal function, but this is less
than that produced by PCNL.
Neal et al. confirmed that consecutive bilateral SWL treatments produced a signifi-
cant overall loss of effective RPF among infant Rhesus monkeys treated with high dose
18 kilovolts (kV), 2000 shocks as opposed to the ones treated with low dose 15 kV, 1500
shocks (125). Bilateral synchronous SWL has also been reported to be relatively safe and
without risk of renal failure or deterioration of renal function in patients with bilateral
urolithiasis. No clinical difference in the long-term effect on renal function, as measured
by serum creatinine, was found in patients with bilateral renal calculi treated with SWL
in a simultaneous vs a staged fashion (126). Perry et al. (127)found bilateral synchronous
SWL to be safe and effective monotherapy for bilateral urolithiasis. There was no renal
function deterioration as per mean serum creatinine, which was found to be similar
preoperatively and postoperatively (1.46 and 1.41 mg/dL, respectively, p = 0.73). Cau-
tion should be warranted as to proper patient selection to avoid bilateral obstruction and/
or acute renal insufficiency. Serum creatinine is not the most accurate measure to evalu-
ate follow up renal function status because one can lose up to 20% of renal function
without significant change in the serum creatinine levels.
S
OLITARY KIDNEY
Contrary to the previously cited studies, and despite the fact that there is a mild
decrease in GFR in patients with solitary kidneys undergoing SWL, other studies have
found SWL to be safe enough to be considered the treatment of choice (128–130). Liou
and Streem (131) also found no evidence that SWL, PCNL, or the combination of both
techniques resulted in the deterioration of renal function in patients with a solitary kidney
after a mean follow up of 53 mo. They concluded that any of these three treatment
modalities are equally efficacious for preserving renal function. Because patients with
solitary kidneys do not have a contralateral kidney that could compensate for the recov-
ery of function of the treated kidney, long-term reduction in renal function is thought to
occur secondary to the presence of multiple renal stones and repeat SWL.
R
ENAL INSUFFICIENCY
SWL in patients with stone-related renal insufficiency has also been addressed. The
combination of ureteral stenting followed by phased SWL allowed these patients to
achieve variable levels of improvement in the renal function (132). Renal transplant
patients can be safely managed with SWL. It is considered the treatment of choice for
calyceal stones sized 5–15 mm in allograft kidneys (133). As in cases of lithiasis in the
mid ureter, SWL in allografted kidneys is performed in the prone position for better stone
localization and treatment.

Chapter 27 / Complications of Urinary Stone Surgery 523
Nonrenal Complications
ADJACENT ORGANS
As the availability of SWL has increased, so have reports of shock-induced injuries
to adjacent soft tissue and organs while the shockwaves travel toward the target organ.
Although infrequent, unusual cases of gastric, splenic, pancreatic, colonic, cardiac, and
vascular complications have been reported in the literature. Urinary fistulae and urino-
mas have also been reported.
Subcapsular splenic hematomas and even splenic rupture have occasionally been
reported (134–136). Subcapsular splenic hematomas can occur either immediately after
SWL or months later. Conservative management has been advocated in selected cases
of subcapsular splenic hematomas, although delayed splenectomy and even death from
an infected hematoma and septic shock have been described (137). These effects have
occurred in patients with portal hypertension with severe coagulopathy and splenom-
egaly. Shockwave-induced injury to pancreatic tissue produces elevation of both serum
and urinary amylase as well as of serum lipase (138,139). Acute pancreatitis has been
described after right side, left side, and bilateral renal or upper ureteral SWL. Although
seldom clinically significant, cases of severe acute pancreatitis have occurred (140).
F
ISTULAE
Infrequent cases of urinary fistulas, pyelocutaneous, ureterocolic, and ureterovaginal
have been described to occur after SWL (141–143). Both pyelocutaneous and uretero-
colic fistulas were related to cases of xanthogranulomatous pyelonephritis (141,142).
Post-SWL urinoma secondary to rupture of the renal pelvis and renocutaneous fistula
complete these groups of urinary collecting system injuries (144,145).
Cardiac arrhythmias, primarily premature ventricular contractions (PVCs), occur in
up to 60% after nonelectrocardiogram-gated SWL (146–149). PVCs are usually not life
threatening, and rapid resumption of the normal cardiac rhythm is achieved by synchro-
nizing the SWL to the patient’s electrocardiogram. SWL has safely been used in patients
with aortic and renal aneurysms (146–148). Although cases of dissection and rupture of
calcified abdominal aortic aneurysms have been reported (150–152), they are probably
secondary to shockwave induced intimal injury. Using a tubless lithotriptor and with the
patient in the supine position, Thomas et al have shown that real-time abdominal ultra-
sound can help to monitor the aneurysm during the shockwave lithotripsy procedure
(153). Caution should then be taken in patients with urolithiasis and calcified abdominal
aortic aneurysms to properly focus the calculus at F2 and to not exceed the recommended
number of shockwaves administered.
F
ERTILITY
Several studies have addressed, in both animals and humans, the effects of SWL
regarding male and female reproductive function and fertility. Changes in DNA histo-
grams resolved within 9 mo, but no statistically significant difference in the semen
analysis, testosterone, or follicle-stimulating hormone was found after studying the
effects of shockwaves on primate testicles (154). In humans, microscopic hemospermia
and a transient decrease in sperm density and motility have been found after SWL to
distal ureteral lithiasis (155). No histological differences were found in shockwave-
treated rat ovaries when compared to control, nor were the pregnancy rates different

524 Urena, Mendez-Torres, and Thomas
among unilaterally oophorectomized rats subjected to direct, indirect, or no shockwaves
(156). Women of childbearing age, after receiving SWL to the distal ureter, were retro-
spectively found to have normal fertility and to have children without chromosomal
anomalies (157). No long-term sequelae or toxicity after SWL seems to exist in male or
female gonads. SWL should not be used in pregnant women to avoid risks of miscarriage
and to avoid shockwaves and/or radiation to the fetus.
Children
The short- and long-term effects of SWL on the pediatric population have also been
studied. Thomas et al. (158) reported no statistically significant effect on linear body
growth or renal function among children with urolithiasis treated with SWL. Although
it has been found that SWL induces transient functional damage of tubular function in
children, as per urinary enzymes activity that returns to baseline within 15 d (159), it is
considered an efficacious and safe treatment of upper tract urolithiasis in children with-
out producing signs of damage to the growing kidney (160,161). Howover, synchronous
bilateral SWL may be inappropriate in the pediatric age group, because studies on infant
primates showed the deleterious effects of synchronous bilateral SWL treatments (125).
The usual recommendation is to limit the amount of energy (such as kilovolts used) and
the number of shockwaves used for treating pediatric urolithiasis.
Hypertension
Since its advent in 1980, the issue of SWL-induced changes in blood pressure has been
controversial, and concern has surfaced as to the long-term risk of the development of
hypertension. Abrupt onset of transient hypertension has been reported in association with
a compressive perirenal hematoma. However, it is unclear whether the late occurrence of
permanent hypertension is caused by SWL alone or the presence of nephrolithiasis, which
by itself increases the relative risk of developing hypertension, or if it is related to aging
of the patient, or multifactorial as a result of the combination of all of these factors.
In 1987, retrospective studies by Newman et al. (162) and Lingeman et al. (163)
independently reported an excessive incidence of hypertension in 8% of patients follow-
ing SWL after a 1-yr follow up. Similarly, Williams et al. (164) and Montgomery et al.
(165) reported that 8% of treated patients developed severe hypertension within 21 mo
after SWL and required treatment 12–44 mo after renal SWL on the Dornier HM-3. This
study was of significant importance because it exceeded the annual incidence of new
onset hypertension in men aged 30–60 yr, which was calculated to be 3–6% (166).
Although this initial finding of an association between hypertension and SWL was
alarming, other studies did not support this association. Yokoyama (167) found that in
patients treated on the Dornier HM-3, after 18 mo an annualized increase in diastolic
pressure and new onset of hypertension to be 0.78 mmHg and 0.65%, respectively.
Significant elevation of diastolic pressure was noted in patients who received a larger
number of shockwaves. Lingeman et al. (168), in a study which included 731 patients
who received SWL only, noted the annualized incidence of hypertension (2.4%) did not
differ significantly from that in control patients (4%). Among patients who received
SWL, no correlation was found between the incidence of hypertension and unilateral vs
bilateral treatments, the number of shockwaves administered, the kilovoltage applied, or
the power index (number of shockwaves times kilovoltage). However, as in Yokoyama
and colleagues’ study (167), there was a significant rise in diastolic blood pressure

Chapter 27 / Complications of Urinary Stone Surgery 525
(DBP) after treatment with SWL (0.78 mmHg), which was not found in the control group
(–0.88 mmHg). Later, an extended follow-up of this study after 4 yr reported an annu-
alized incidence of new onset hypertension in SWL patients to be 2.1% compared to
1.6% in non-SWL patients. A statistically significant difference in the annualized DBP
occurred in SWL-treated patients when compared to non-SWL patients. DBP decreased
for both groups following treatment, but the decrease was significantly greater for those
patients whose kidneys were not exposed to shockwaves (169). Claro et al. (170)
also
found the incidence of hypertension after SWL to be similar to that of a normal popu-
lation (3.92%), although the diastolic pressure was statistically higher after treatment.
More recently, Jewett et al. (171) reported the results of a prospective, randomized
controlled trial of normotensive patients presenting with asymptomatic renal calculi.
Patients were randomized to receive immediate SWL or be placed on an observation
protocol, reserving SWL treatment for the onset of symptoms. At 24 mo follow-up, there
was no observed difference in the incidence of new onset hypertension between the
treatment and observation groups (2.7% in the SWL group and 2.5% in the observation
group, for an overall incidence of 2.6%). In a similar study, Elves et al. (172), studied
the changes in BP over a mean of 2.2 yr in patients with small asymptomatic renal calculi
randomized to observation or SWL and found no evidence that SWL causes changes in
blood pressure. On the other hand, Strohmaier et al. (173) prospectively studied the BPs
of stone patients undergoing different types of treatments (SWL, ureteroscopy, percu-
taneous nephrolithotomy/open surgery, spontaneous stone passage, and no treatment).
At a 24 mo follow-up, in all groups, regardless of the stone location and type of treatment,
SBP and DBP were significantly higher than the pretreatment levels, and they concluded
that renal stone disease itself rather than the type of treatment was perhaps responsible
for the increase in SBP and DBP.
Although renin-mediated hypertension (renovascular hypertension) has been asso-
ciated with renal trauma, hypertension after SWL has not always been associated with
an increase in renin production. To further investigate their association, studies have
been done in animals and humans. Begun et al. (174) did not observe renin-mediated
hypertension despite the excessive number of total shockwaves (20,000) delivered to
the kidney of each minipig over a 4-mo period. On the contrary, Neal et al. (175) did
find evidence of renin elevation after SWL on rhesus monkeys. Renin levels remained
elevated over baseline in the infant monkeys but returned to normal values in the adult
group of treated animals. In one of the few studies done in humans, Strohmaier et al.
(176) studied endothelin production, which was thought to stimulate renin secretion
and its effect on hypertension after SWL. They found only a slight and transient
increase in active renin, whereas no correlation between endothelin and active renin
was noted. They concluded that the increase in active renin was not mediated by
endothelin and that the transient increase in active renin could not be attributed to the
development of hypertension. Although an increase of renin levels after SWL could
explain in part the early elevation in blood pressure after SWL, its long term occur-
rence cannot be explained solely based on renin production.
Assessment of the intrarenal resistive index (RI) by color Doppler ultrasonography at
the level of the interlobar arteries is a noninvasive diagnostic modality for studying the
renal vascular resistance and its associated changes in the arterial system. The intrarenal
resistive index was originally found to be increased in 30% of patients immediately after
SWL (177). Later, statistically significant elevated resistive index levels were observed
in 45% of patients older than 60 yr who had hypertension after SWL, whereas, in the
