Khine M.S., Saleh I.M. Models and Modeling: Cognitive Tools for Scientific Enquiry
Подождите немного. Документ загружается.

Part I
Theory Formation and Modeling
in Science Education


Chapter 1
Modeling and the Future of Science Learning
Richard K. Coll and Denis Lajium
Introduction
Models and modeling are of such importance in science that the appropriate under-
standing of, and ability to use, models is seen by many authors as central to an
understanding of science (Gilbert & Boulter, 1998; Harrison & Tr eagust, 2000;
Ramadas, 2009). In this chapter we consider key aspects of models and model-
ing. We begin by describing the nature of models and modeling. We then discuss
how models and modeling relate to the nature of science and scientific enquiry. This
followed by a description of modeling as a cognitive tool and consideration of how
models and modeling relate to the learning of science. We conclude the chapter
by highlighting the areas of needed research in science education with respect to
models and modeling.
The Nature of Models and Modeling
Three principal purposes for modeling in the sciences are reported in the sci-
ence education literature: (1) to produce simpler forms of objects or concepts;
(2) to provide stimulation for learning or concept generation, and thereby support
the visualization of some phenomenon; and (3) to provide explanations for scien-
tific phenomena (Gilbert & Rutherford, 1998a, 1998b; Passmore & Stewart, 2002;
Ramadas, 2009; Thomas & McRobbie, 2001). The process of modeling involves
the transfer of some features of the source of the model to the target of the model
(Brodie et al., 1994) (Fig. 1.1). We thus have a target, something we want to under-
stand; a source, something known to us from our everyday life or prior experiences;
and the model which helps us bridge between the two (Brodie et al., 1994; Suckling,
Suckling, & Suckling, 1978).
R.K. Coll (B)
Science and Engineering Faculty, University of Waikato, Hamilton, New Zealand
e-mail: r.coll@waikato.ac.nz
3
M.S. Khine, I.M. Saleh (eds.), Models and Modeling, Models and Modeling
in Science Education 6, DOI 10.1007/978-94-007-0449-7_1,
C
Springer Science+Business Media B.V. 2011

4 R.K. Coll and D. Lajium
Model
SourceTarget
Fig. 1.1 The relationship between a model, its source and target (after Brodie et al., 1994)
#
model (R
m
)
analog (R
1
)
target (R
2
)
analogy (A)
Fig. 1.2 Analogical transfer by structural mapping between two domains (from Duit, 1991)
A useful visualization of such mapping from target to source is provided by Duit
(1991) (Fig. 1.2). In Duit’s representation, the process of modeling involves analog-
ical mapping—many authors believe that all models are in fact analogies and that
all modeling consist of mapping via the use of analogy (A) (see, e.g., Gilbert, 2004;
Harrison, 2008). In this mapping, there are features in parts of the structures of the
analog (R
1
) and target (R
2
) that are shared (R
m
).
From Fig. 1.2 it can be seen that modeling involves s implification of the target.
In other words, the model is a simpler version of the target and shares only some
attributes with the target (i.e., R
m
). As a consequence, the extent to which the target
and source share attributes varies (Maksic, 1990), and because of this the model and
target may in some cases be very different and may be different in numerous ways.
This is an inherent feature of models and modeling and should not be seen as some
sort of flaw or limitation of models; indeed as we shall see it is a key component
of models and modeling. The fact that the model is simpler than the target means
is in fact beneficial, because we can ignore less relevant or unimportant details, and
instead focus on the most interesting or important facets—selected and dominant
features that help us understand key attributes of the target. Consequently, the use
of a model enables scientists to identify key aspects of the target without becoming
distracted by unimportant detail or attributes (Gilbert & Boulter, 1998). The initial
grasping or generation of an abstract concept is more readily achieved when the
concept has been stripped to the bare necessities required for understanding—a pro-
cess of what Weller (1970, p. 118) describes as providing “a basic outline of the
‘forest’.”
So the nature of the target and the purpose of models, and subsequent modeling,
vary, and so the models themselves vary considerably (Gilbert & Osborne, 1980).
1 Modeling and the Future of Science Learning 5
Some are quite simple such as a two-dimensional drawing or a ball-and-stick model
used to illustrate aspects of molecular geometry in a small molecule. Others are
highly complex and might, for example, be used to provide detailed predictions
of physical or chemical properties (e.g., quantum mechanical modeling of atomic
structure, modeling of spectroscopic data such as nuclear magnetic resonance—
NMR).
Although many authors emphasize the importance of models in science, there is
much variation in the use of the terms model and modeling, and as a consequence
numerous typologies of models have been developed. Examples of types identi-
fied in the literature include knowledge models scale models target systems mental
models, and expressed models (see, e.g., Brodie et al., 1994; Gilbert & Boulter,
1998; Harrison & Treagust, 1998; Justi & Gilbert, 1999; Smit & Finegold, 1995;
Suckling et al., 1978). These typologies can, however, be distilled down to a few
key types. These are target models—the concept or phenomena of interest— and
the user’s model—the model possessed or used by an individual, often a mental
model (Norman, 1983). A users’ mental model is a personal mental construct and,
according to radical constructivists, such a model is only accessible by another up
to a point (Gilbert, 2004; von Glasersfeld, 1993). When users’ models or mental
models are shared and aspects of these are agreed they become consensual models
(and after time an historical model, see Gericke & Hagberg, 2007). A consensus
or historical model is a model that has been subject to some form of “testing” (e.g.,
physical testing or argumentation) by a particular social group. In the case of science
this group is the scientific community, and this group or community agrees on the
attributes and value of the model. Teaching models, as the name implies, are mod-
els using during instruction. Expressed models are models as they are expressed,
or communicated, to others. This may take the form of actual expression (e.g., in
language or drawings), or as one can interpret the model from analysis of presented
models (e.g., in textbooks or scientific writing). This notion recognizes a model
as the construct of an individual, or a particular community of practice, consistent
with the view that any model is a mental model (Gilbert, 2004), and thus a personal
mental construct (von Glasersfeld, 1993).
The above description of the nature of models is driven by the purpose or use of
the model. Models also may be classified on the basis of the nature of the model.
Obvious examples are physical models and mental models; but there also are numer-
ous other types of models identified in the science education literature. Interestingly,
the model typologies based on model nature or type throw up some things that we
may not have thought of as models, for example, chemical equations. But if one
considers the nature of models (viz, they are a representation of an entity or phe-
nomena), then such classification is logical and appropriate. Again, classification
schemes can be summarized in broad terms. So models have been classified on the
basis of their nature as falling into two broad types physical models and concep-
tual/symbolic models (Brunner, 1967; Coll, 1999; Suckling et al., 1978). Physical
models include things such as concrete physical models such as scale models used
in engineering design or architecture and ball-and-stick or space-filling models used
in chemistry (Black, 1962;Harré,1970; Smit & Finegold, 1995; Tomasi, 1988
).
6 R.K. Coll and D. Lajium
Conceptual models consist of diagrams such as reaction schemes and metabolic
pathways (Gilbert, 2004; Harrison & Treagust, 1996), and formulae (Gilbert, 2004;
Harrison & Treagust, 1996; Trindle, 1984). Some models, like analog models, may
be physical or conceptual/symbolic (Black, 1962; Duit, 1991; Peierls, 1980; Tomasi,
1988). Conceptual/symbolic models represent mental constructs; in other words,
they are mental models (Brunner, 1967; Suckling et al., 1978) or expressed mental
models (Gilbert, 2004).
Models, Modeling, and the Nature of Science
Here we delve into the nature of models and modeling in a little more detail,
and consider how this relates to the nature of science. As noted above models
possess a number of fundamental properties, a key feature being that they are
human inventions or (mental) constructions. As such, they represent an approxima-
tion of reality (Portides, 2007), or a limited “version” of the target, and inevitably
present an incomplete understanding of the entity or phenomena (Carr & Oxenham,
1985; Ganguly, 1995; Greca & Moreira, 1997). We might say they are “wrong”
or “limited” in some key aspect (Tomasi, 1988; Trindle, 1984). Here, we mean
they are inherently limited, because human understanding can never match real-
ity (Harding & Hare, 2000). But many models possess notable limitations that are
clearly recognized in advance, and these limitations may be unhelpful in the use
or function of the model. Two examples illustrate our meaning here. First is the
Aufbau principle used to explain the electron configuration of the elements. It is
well recognized that many elements do not agree with predictions of the Aufbau
principle (e.g., chromium and copper). But it “works” for most elements, so it is
retained, and explanations are developed for the anomalous elements (e.g., inter-
electron repulsion, Zumdahl & Zumdahl, 2003). Perhaps a more dramatic example
is ligand field theory. Ligand field theory models bonding in organometallic com-
plexes and is based on simple electrostatic attraction between a metal center and
electron-rich ligands (atoms, ions, or groups) that surround it. This attraction leads
to perturbation of energy levels for the metal d orbitals in particular, and the model
allows for predictions of electron distribution and bonding. A major use of model
is in the interpretation of electronic absorption spectra in the UV–visible portion
of the electromagnetic spectrum. However, there is much evidence to suggest that
ligand field theory fails in many ways; the simplest example relates to the failure of
ligand field theory to explain the spectrochemical series. Interestingly, despite these
obvious, and fairly serious, limitations, ligand field theory is still widely used by
modern scientists (see, e.g., Nakamoto, 2009); simply because in many instances it
is able to correlate some experimental data adequately, and it is therefore useful in
some circumstances.
What is of particular interest here, to our discussion of the nature of science, is
that even when models like ligand field theory possess severe limitations this does
not necessarily mean that they are automatically discarded—particularly if not eas-
ily replaced (students often think once a model fails, it is discarded, see Shen &
1 Modeling and the Future of Science Learning 7
Confrey, 2010). It is this highly pragmatic use of models that are known to pos-
sess quite significant limitations that distinguishes scientists from students (Shen &
Confrey, 2010; Williamson & Abraham, 1995). Hence, a key aspect of the nature
of science, as it pertains to models and modeling, is that scientists understand the
nature of models and see models in a very functional, utilitarian, manner (Portides,
2007). They instinctively, or as a result of their training, recognize that models
are intended to serve the user and frequently require modification (e.g., when new
experimental data are obtained, see Borges & Gilbert, 1999). Grosslight, Unger, J ay,
and Smith (1991) observe that one reason for this difference between scientists and
students is that students and non-scientists tend to think of models in concrete terms,
effectively as scale models of reality (see also, Abell & Roth, 1992; Bent, 1984b;
Carr, 1984; Clement, 1998), and thus do not appreciate their limitations (Ogan-
Bekiroglu, 2007). This, it is argued, occurs because some well-known models have
proven spectacularly successful. Examples include the Watson–Crick model for t he
structure of DNA (Rodley & Reanny, 1977; Watson & Crick, 1953), Einstein’s rela-
tivity (Clark, 1973; Kline, 1985), and Schrödinger’s wave-mechanical model of the
atom (Kline, 1985; Moore, 1989). What this may mean is that successful models
are so powerful at explaining well-understood observationsthat they end up being
regarded as “facts” (Schrader, 1984). In other words, a student or novice may con-
fuse a highly successful, well established, model with reality, or the target it is being
used to model.
Another aspect of the nature of science and models is the fact that it is com-
mon for scientist to use multiple models to describe an entity (Flores-Camacho,
Gallegos-Cázares, Garritz, & García-Franco, 2007) or explain phenomena/data
(Barnea, Dori, & Finegold, 1995; Birk & Abbassain, 1996; Lin & Chiu, 2007). The
use of multiple models is particularly prevalent in some sciences such as physics
and chemistry, especially when it comes to developing an understanding of abstract
microscopic concepts like atomic structure and chemical bonding (Brodie et al.,
1994; Chiu, Chou, & Liu, 2002; Comba & Hambley, 1995; Eilam, 2004; Glynn &
Duit, 1995; Lin & Chiu, 2007; Lopes & Costa, 2007). Again, as might be expected,
there are significant differences in how scientists view and use multiple models (see,
e.g., Clement, 1998; Flores-Camacho et al., 2007; Grosslight et al., 1991; Harrison
& De Jong, 2005), with scientists again acting in a highly pragmatic fashion. As
an illustration, there are numerous models for chemical bonding, and scientists
use whichever model seems appropriate and convenient (Coll & Treagust, 2003a,
2003b). Consider reactions of aromatic chemical substances. Molecular orbital the-
ory provides the most comprehensive explanation for the bonding in aromatics (viz,
delocalization of electron density across the molecule), but scientists routinely use
electron dot Lewis structure-type notations and formulae when presenting reaction
schemes (Coll & Treagust, 2002a). Key here, again, is that the scientist retains in his
or her mind an acute appreciation of the limitations that such simple models possess,
and that there are often apparent “contradictions” between models (Flores-Camacho
et al., 2007) because they are used for different purposes.
The final aspect of models related to the nature of science we wish to raise here is
the success or otherwise of models. We have already noted that all models possess
8 R.K. Coll and D. Lajium
some limitations or fail in some way, that is, the very nature of the models and mod-
eling. We also suggested above that scientists still use these models, in ways they
find useful. Maksic (1990) makes an interesting point, noting that a good match
between the predictions of a given model and experimental data indicates that a
model is reliable and useful. But Maksic goes on to observe that this should not
be taken to infer that complex and sophisticated quantitative models are necessarily
superior to simpler qualitative counterparts. Simple qualitative models, despite their
apparent “lack of accuracy” or lack of sophistication, possess considerable value in
science, for example, in theory generation (Koponen, 2007). Likewise, construct-
ing an analogical model allowed Maxwell to gain access to a systematic body of
knowledge, consisting of a structure of causal and mathematical relationships, dur-
ing the development of his famous equations (Nersessian, 1992; Franco, de Barros,
Colinvaux, Krapas, Queiroz, & Alves, 1999).
Models and Modeling in Scientific Enquiry
The literature thus suggests that models and modeling are an important part of doing
science and that models play a key role in scientific enquiry. Models can play a
number of roles in scientific enquiry, and four distinct functions have been identi-
fied: discovery development evaluation, and exposition (Cosgrove, 1995; Holyoak &
Thagard, 1996, 1997). Discovery occurs when model contributes to the formation or
generation of a new knowledge or the formulation of a hypothesis (Ganguly, 1995;
Johnson-Laird, 1989; Stavy & Tirosh, 1993). When a hypothesis or discovery has
been made, the model may then be used to further its development—either from
a theoretical or from an experimental perspective. As the model is developed, we
need to test the model, and so models play an important role in the evaluation of a
hypothesis, and in evaluating arguments for or against the hypothesis. Finally, mod-
els are commonly used for exposition, that is, they are used to explain hypotheses
or theories to others.
Some authors argue that all models are analogy; whether one believes this or
not, analogies are an important model type used for concept generation. Because
such analogy or model use in concept generation is a personal mental process, it
is difficult to document, although there are numerous scientific theories for which
models clearly contributed to some vital stage of a scientists’ thinking, whether it
was in discovery or the development of an idea (as described above). There are
a number of well-known models that clearly illustrate the role that models can
play in concept generation during the development of scientific theories (Sutton,
1993). Holyoak and Thagard (1996) identified a number of models that they say
have played a role in theory development (mostly analogies). Examples include the
Kekulé’s snakes biting their tails used to model the structure of benzene. Model use
thus plays a generative role—mostly when in situations for which prior knowledge
is weak or when there is little connection between ideas (Silva, 2007; Wong,
1993a, 1993b). The generative function here comes from the way we use models
(see Duit’s model described in Fig. 1.2 above), in that the scientist, trying to solve
1 Modeling and the Future of Science Learning 9
a problem or explain some data, engages in the mapping of attributes from the
known to the unknown (Harrison, 2008; Wong, 1993a). In doing so, the analogy or
model provides insights and inferences. As Wong sees it, these generative models
are dynamic tools rather than static representations used for understanding. In such
circumstances, individuals seek to find ways of making it easier to explain observed
phenomena and so develop their own models to advance their understanding (see
also, Johnson-Laird, 1989).
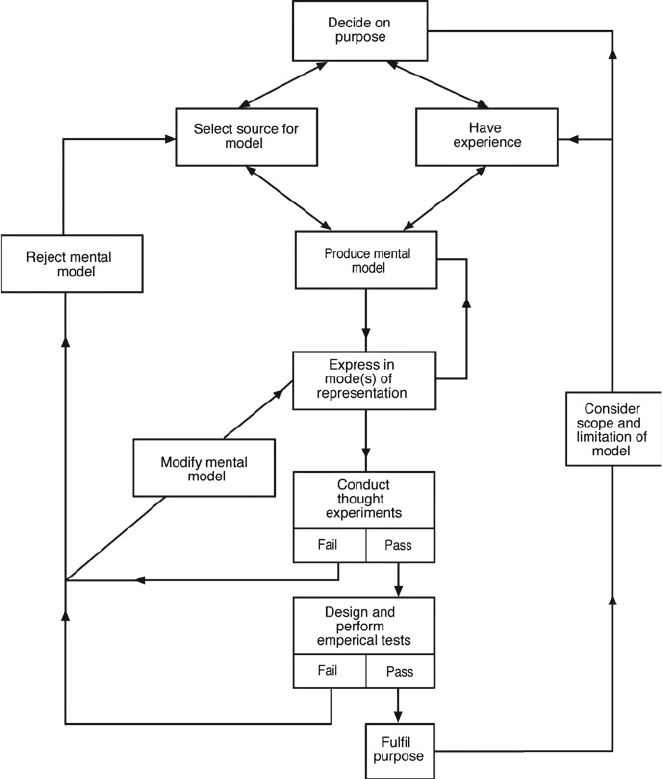
Justi and Gilbert (2002) analyzed how models are produced in science and devel-
oped a “model of modeling” (Fig. 1.3). This model of modeling exemplifies the
Fig. 1.3 A “model of modeling” (after Justi & Gilbert, 2002)
10 R.K. Coll and D. Lajium
essential features of scientific modeling as used in scientific enquiry. Notable is
the dynamism of the model (Maia & Justi, 2009), in that data are “reconsidered”
constantly, and the relationship between data and the mental models developed
by scientists to explain these data is examined in an on-going, iterative fashion.
Considerable emphasis is placed on testing any scientific model and re-examination
of relationships between data and models (as we might expect). Acceptance or
rejection of a model is related to the purpose of the model, which remains
paramount—consistent with the literature described above. Sins, Savelsbergh, van
Joolingen, and van Hout-Wolters (2009) sum the use of models and modeling in
scientific enquiry nicely, describing it as a process of developing, comparing, and
testing competing models.
Modeling as a Cognitive Tool
The above discussion indicates that models, particularly analogical models, func-
tion as cognitive tools. The literature also suggests that students fail to appreciate
that the principal function of mental models in science is to aid in the understand-
ing of some phenomenon, as mentioned above. This lack of insight is highlighted
in studies across different science disciplines. For example, studies by Grosslight
et al. (1991); Abell and Roth (1992) in the biological sciences, Bent (1984b)in
chemistry, and by Raghavan and Glaser (1995) in physics support this conclu-
sion. Gabel, Briner, and Haines (1992) suggest that mismatch between scientists’
and students’ perceptions of the role of models may be due to the inability of
novices to operate at the formal level. To illustrate, chemistry can be described
on three distinct levels, sensory, atomic/molecular, and symbolic. Usually a chem-
ical substance is represented on the atomic/molecular level using models such as
three-dimensional representations (Ke, Monk, & Duschl, 2005). However, students
typically attempt to address chemical problems on one level alone, for example,
using a purely symbolic approach (Jansoon, Somsook, & Coll, 2008, Jansoon,
Coll, & Somsook, 2009; Tan et al., 2008). Consequently, some authors argue that it
is essential for instructors to design problem-solving exercises that utilize all three
levels in an integrated manner to foster genuine understanding, as opposed to merely
achieving competence in the use of algorithms (Gabel et al., 1992; Green, 1996;
Jansoon et al., 2008, 2009; Nakhleh, Lowrey, & Mitchell, 1996; Pestel, 1993), or
to involve students in concrete sensori-motor experiences (Ke et al., 2005) that pro-
vide a range of experiences that develop p-prims that can be incorporated into their
schema.
Given the complexity of some specific models, the huge variety of models, and
the issues associated with model use, it is not surprising that many authors regard the
appropriate use of models as a complex task. Bent (1984a) makes parallels between
learning chemistry via models and learning a language. Hence, the literature recom-
mends that science teachers introduce a model only when necessary to explain an
experiment or an observation (Coll, France, & Taylor, 2005; Harrison & Coll, 2008).
Students appreciate more about how scientists actually use models when led through
