Khanna A.S. (Ed.) High-Performance Organic Coatings: Selection, application and evaluation
Подождите немного. Документ загружается.

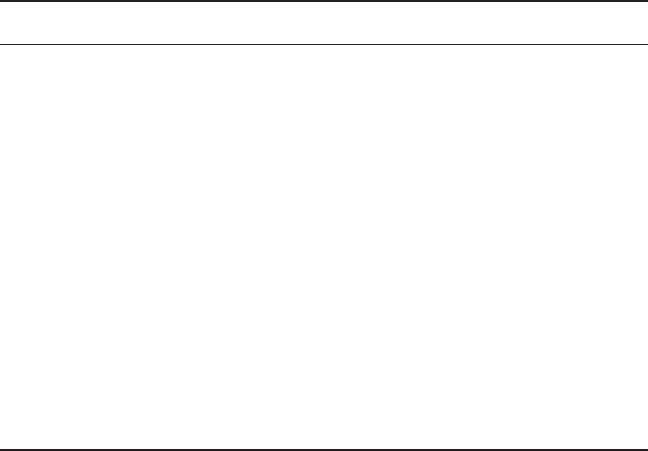
266 High-performance organic coatings
13.8.1 Co-solvents in waterborne coatings
Although water is an environmentally friendly solvent for the coating
industry, it requires some additives to make it suitable for waterborne coat-
ings owing to its unique chemical and physical nature. In most waterborne
systems, the inclusion of some co-solvent is necessary to improve coales-
cence, freeze–thaw stability, levelling and wetting. Solvents speed up water
release, adjust the drying time, assist pigment dispersion and control
foaming. Some of these co-solvents and their functions in waterborne
coating formulations are listed in Table 13.6.
13.8.2 Surfactants
The constituent particles of liquid and solid, which may be ions, atoms or
molecules, in bulk are attracted to their neighbours on all sides, whereas
those in the surface layer are attracted on only three sides. This results in
an unbalanced force, known as the surface tension. The establishment of
this surface tension in water causes the adoption of the minimum possible
area (a spherical shape) by the water droplets, which results in poor wetting
of the substrate by waterborne coating formulations. Therefore, a water-
borne coating requires some surfactants to reduce the surface tension of
the coating, which improves the wetting of the substrate. Surfactants may
Table 13.6 Function of co-solvents in waterborne coatings
Solvent properties Function Example
Coalescing agent for
latex
Reduces glass transition
temperature (T
g
) of
resins
Hexylene glycol,
n-methyl-2-pyrrolidone
Freeze/thaw stabilizer Depresses the freezing
point of water
Alcohols, non-ionic
surfactants
Levelling aid
mobility
Propylene glycol
Wet edge extender Reduces viscosity of
paint and evaporation
rate of solvent
Propylene glycol,
diethylene glycol
monoethylether
Defoamer Imparts low surface
tension to foam cell
wall
Octanol, pine oil,
mineral spirits
Dispersant Provides good wetting
and dispersion of
pigments
n-Methyl-2-pyrrolidone
Prolongs wet film
© 2008, Woodhead Publishing Limited
Waterborne coatings for corrosion protection 267
also be added to aid substrate wetting, to assist stabilization of the latex or
to maintain dispersion of pigments. There are three major functions of
surfactants:
• To improve substrate wetting
• Stabilization of latex
• Proper dispersion of pigment.
Surfactants consist of polar (hydrophilic) and non-polar (hydrophobic)
moieties. The non-polar moiety experiences a repulsive force by water
molecules, which leads to the relative depletion of surfactants from the bulk
of the liquid water and accumulation at the surface where they have to
reduce surface tension. Thus, in the presence of surfactants, the surface
energy of the interface will have a lower energy state than in the bulk.
Surfactants are divided into three groups: anionic, cationic and non-ionic
(Fig. 13.15). Anionic surfactants possess a negative charge in solution and
agents, whereas cationic surfactants possess a positive charge in solution
and are typically used as antibacterial agents, fabric softeners, corrosion
is quaternary ammonium compounds, R
4
N
+
, though in coatings their use is
limited.
However, these surfactants are present in the paint formulation but are
not reactive (no covalent bonding with polymeric particles) in the polym-
erization process. They result in the destabilization of the paint formulation
under high ionic strength, freezing and high shearing. Poor adhesion, water
sensitivity and low-dimensional stability have also been observed when the
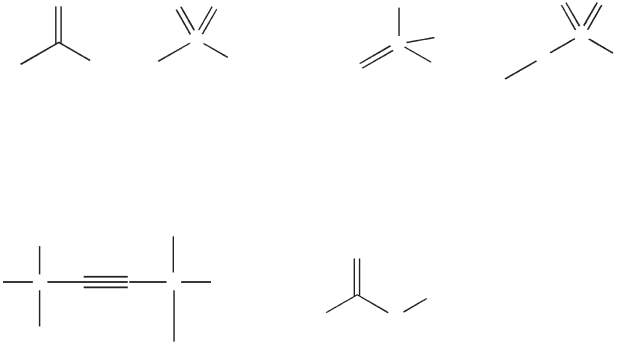
meric surfactants. Reactive surfactants are able to participate in one of the
chemical reactions involved in the polymerization process. The other way
to avoid the desorption of the surfactants from the particle surface is to use
Guyot [11] reviewed the performance of reactive surfactants and their
applications in the synthesis of latexes for waterborne coatings. A large
number of reactive surfactants, non-ionic, anionic, cationic or even
zwitterionic (Fig. 13.16), have been prepared and tested in emulsion, mini-
emulsion, micro-emulsion or dispersion polymerizations, of styrene and
acrylic monomer(s), in batch or semi-batch processes, to prepare both
homopolymers and core-shell copolymers. A novel wetting agent, sodium
are typically used as soaps, detergents, emulsifiers, dispersants and wetting
film was exposed to water or high conditions of humidity. The bad perfor-
mance of film is due to desorption of surfactant from the particle surface
or migration towards the film surface, and formulation of hydrophilic
domains within the film upon phase separation. There are two possible
ways to avoid these difficulties: by using either reactive surfactants or poly-
polymeric surfactants that are very difficult to desorb.
inhibitors, ore flotation additives, emulsifiers and dispersants. One example
© 2008, Woodhead Publishing Limited
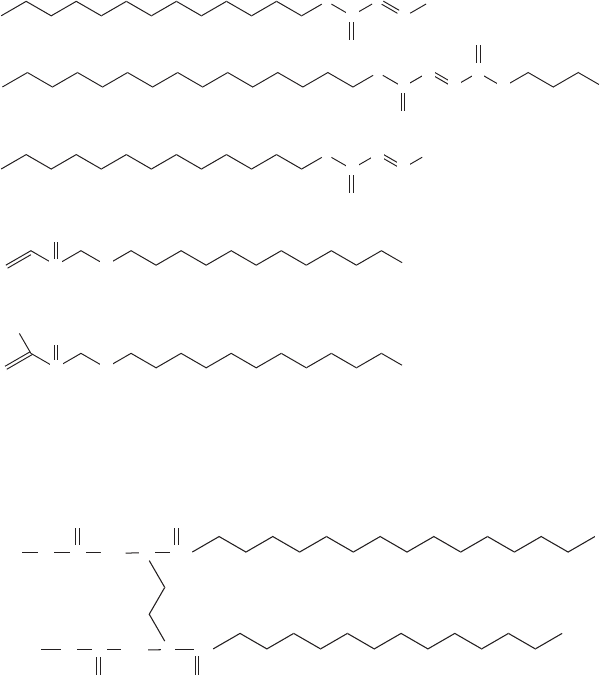
268 High-performance organic coatings
salt of N,N-dipalmitoyl-ethylenediamine-diacetic acid (Fig. 13.17), was syn-
thesized by Bi et al. [12].
ogy is essential for the stability of the paint, the manufacturing process
(pigment dispersion) and transport through pipelines as well as for its
application characteristics. Thixotropes or rheological control agents for
waterborne coatings are as important as those for solventborne coatings.
The viscosity of latex systems is generally very low and in consequence
such systems require viscosity builders. Viscosity builders include water-
Anionic surfactant
Non-ionic surfactants
Hydrophobic surfactants
Linear and branched aliphatic hydrocarbons
Alkylbenzene
Alkylnaphthalenes
Alkylphenols
Polyoxypropylenes
Silicones
Fluorocarbons
Acetylene glycol Alkanolamide
Carboxylate Sulphonate SulphatePhosphate
O
C
C
OH
OH
OH
O
N
H
H
H
R
R
R
O
O
O
O
OO
S
P
OR
R
OR
S
O
–
O
–
O
–
O
–
13.15 Common surfactants used in waterborne coatings.
13.8.3 Rheology modifier
Rheology (flow properties) is the study of the deformation and flow of
matter under the influence of an applied stress. The control of paint rheol-
© 2008, Woodhead Publishing Limited
Waterborne coatings for corrosion protection 269
O C
H
H
H
C
COONa
H
O
Hemiester (sodium salt)
O
H
O
O
Alkyl maleic sulphonate
SO
3
Na
Methacrylol sulphate
O COONa
H
O
Undecanylol sulphate
SO
4
Na
O
Crotonoylol
SO
4
Na
O
CH
3
Hemiesterethoxylate methyl ester (sodium salt)
C
O
C
C
C
C
C
C
C
CO
CO
13.16 Reactive surfactants.
Na
O
C
CH
2
N
C
OO
Na O C
CH
2
N
C
O
O
Sodium salt of N,N′-dipalmitoyl-ethylenediamine-diacetic acid
13.17 A novel wetting agent.
soluble high molecular weight polymers such as cellulosic, alkali-activated
polymers, such as those based on acrylic acid, ethoxylated urethane-based
associative thickeners, and clays such as attapulgites. A list of some com-
monly used thixotropes and their functions is given in Table 13.7.
13.9 Film formation
13.9.1 Film formation by water-soluble polymer
Film formation involves the deposition of a polymeric solution onto a sub-
strate and its transformation into an adherent solid coating as the water
dries out. During the application process, polymer molecules tend to
become stretched, owing to the following:
© 2008, Woodhead Publishing Limited

270 High-performance organic coatings
• The energetically favourable interaction between the polymer segments
and the water molecule, which may be maximized by uncoiling of the
polymer.
• The presence of mutual forces of repulsion in polymer.
Once the coating process is complete, the polymer molecules become
state of high shear exists within the coating solution during the coating
application.
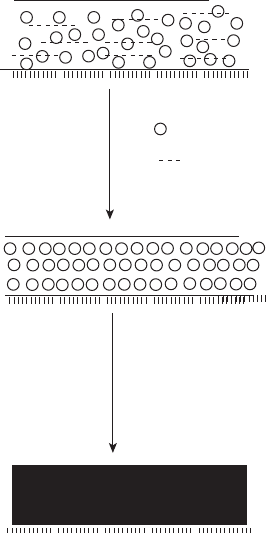
13.9.2 Film formation by latex paint
Fig. 13.18.
1. Evaporation of water by leaving behind the polymer, pigment and other
constituents of paint on the substrate.
formance of the resulting coating.
Thixotrope Example Properties
Cellulosic polymer Methyl cellulose, hydroxyethyl
cellulose, hydroxy propyl
cellulose
Increases viscosity of
latex
Polyacrylate Polyacrylic acid, polymeth-
acrylic acid and their salts
Not prone to microbial
attack
Anionic thickener Anionic polycarboxylic acid No microbial attack
Non-ionic
thickeners
Ethoxylated polyether-based
urethane block co-polymer
Thicken by means of
secondary valency
association
Clay-type
thickeners
Appapulgite clays (anti-
microbial attack)
Impart viscosity by
creating a network of
Fibrous
thixotropes
Cellulosic and polyaremid
Table 13.7 Rheology modifiers in waterborne coatings
fibres
free to return from a stressed state to their original configuration, as long
as they have not reached their elastic limit, and form an even film, since a
The mechanism of film formation in latex paint is more complex than for
paints derived from true polymeric solutions. Once the film has been spread
on the substrate, drying of the film starts. It involves two steps as shown in
particles take place for film formation. In this step, full incorporation of
the pigment particles into the film is crucial for assuring excellent per-
flocculated needles
2. Once the water evaporates, flattening and coalescing of the polymer
© 2008, Woodhead Publishing Limited
Waterborne coatings for corrosion protection 271
a number of factors such as the natural hardness of the polymer, the pres-
ence of coalescing solvent in the latex, and electrostatic repulsive interac-
tion at the surface of the polymeric molecules which themselves depend on
while others form powdery layers. Commonly, loss of water by evaporation
transition temperature and the particle size of the polymer components of
13.10 Application methods
The application technology for waterborne coatings is comparable to that
of conventional solventborne coatings. If a facility is using a water wash
booth, overspray is easily recovered and reused if colours are appropriately
segregated. Uncured waterborne coatings can be cleaned from equipment
Latex
Substrate
Polymer particle
Water
Application of latex
Evaporation of water and approach of
polymeric particles towards each other
Generation of small capillaries of
water between polymeric molecules
13.18 Film formation in latex paint.
Polymeric film on substrate
the temperature of film formation. The temperature at which latexes cast
continuous and clear film is called the minimum film formation tempera-
ture. When drying, some latex paints produce continuous and strong film
results in a powdery finish to the coating, which has no cohesion. The glass
the latex decide the minimum film formation temperature [1].
The rate of flattening and coalescing of polymer particles is influenced by
© 2008, Woodhead Publishing Limited
272 High-performance organic coatings
with water. Electrostatic spray can be used if the electrically conductive
waterborne paint is isolated from the electrostatic system. However, some
formulations or substrates might require special pumps and piping to
prevent corrosion from water in the formulation. In addition, for product
complete cure in a reasonable period of time.
13.11 Performance evaluation of waterborne coatings
Selection of coating recipes depends on several factors such as the desired
life of the coating, the nature of the substrate, and climatic conditions.
There are several laboratory tests in use to demonstrate the protective
behaviour of coatings and to give reliable predictions of their lifetime. It is
generally expected that the test environment should be the same as or
similar to that of the actual application. However, such a natural exposure
test requires too long a time. For reducing the test time, accelerated natural
exposures and laboratory tests have been developed. Commonly used
accelerated tests such as salt spray, accelerated weathering, humidity, tem-
perature and different contamination conditions, are traditionally used to
assess the anticorrosive behaviour of the paint coatings.
Like solventborne coatings, waterborne coatings are also available for
high-performance applications such as corrosion protection, abrasion resis-
tance, UV stability, thermal stability etc. Various waterborne coatings
derived from conventional polymers have been well discussed in various
texts [1, 2]. A number of review articles are available on sol-gel based coat-
ings [1, 14] but only a few address waterborne sol-gel products [15, 16].
Today sol-gel coating is one of the most promising high-performance water-
borne coatings. In this section, we will discuss the performance of water-
borne sol-gel coatings specially developed by our group.
13.11.1 Corrosion resistance
Excellent corrosion resistance is one of the most desirable properties of
high-performance coatings. Potentiodynamic polarization, electrochemical
impedance spectroscopy and salt spray tests are traditionally used to
assess the anticorrosive behaviour of paint coatings. Waterborne sol-gel
coatings based on methyltrimethoxysilane (MTMS) and 3-glycidoxyprop-
yltrimethoxysilane (GPTMS) were developed [13]. Two amino-silanes,
namely γ-aminopropyltriethoxysilane (1N), N-(β-amino ethyl)-γ-aminopro-
pyltrimethoxysilane (2N) and hexakis(methoxymethyl)melamine (HMMM),
were used as crosslinking agents. Aluminium alloy AA6011 was dip coated,
dried at room temperature and placed in a furnace at 120°C for 30 min for
finishing, coatings need to dry or cure at elevated temperatures to ensure
final curing. The anticorrosive behaviour of the coatings in 3.5% NaCl solu-
© 2008, Woodhead Publishing Limited
Waterborne coatings for corrosion protection 273
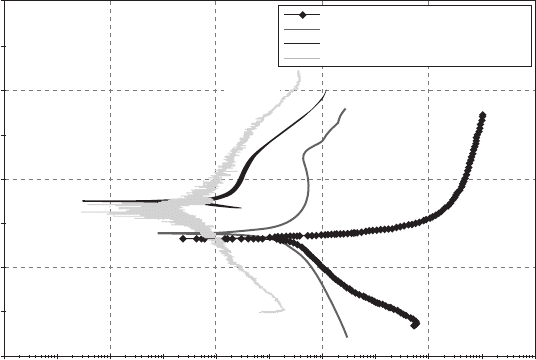
tion was found to be excellent. The corrosion currents of the coated alu-
minium alloy decreased by two orders of magnitude compared to bare
aluminium alloy, which implied that the coating forms a dense barrier
against penetration of water and chloride ions (Fig. 13.19).
It was found that the anticorrosion ability of various crosslinkers is in the
order HMMM > 2N > 1N. Addition of p-TSA as catalyst in the 1N coating
tance. The shifting of open circuit potential towards the noble direction, as
compared with the uncatalysed 1N coating, and the reduction in the corro-
sion current from 0.67 × 10
−6
A to 1.26 × 10
−7
of the addition of the p-TSA as catalyst (Fig. 13.20).
Although organosilanes exhibit excellent corrosion resistance, their use
reduction and easy handling of sol-gel materials have become important
issues for manufacturers of paint raw materials, paint users and researchers.
One solution to this problem is the incorporation of conventional organic
polymers in silanol sol.
In order to reduce the cost, conventional waterborne alkyd and polyester
resins were incorporated in the MTMS–GPTMS sol. These conventional
organic polymer-incorporated sol-gel coatings have shown excellent
corrosion resistance on aluminium and magnesium alloys. The impedance
spectra of bare and polyester-incorporated MTMS–GPTMS coated
0.0000000001
–2
–1
0
HMMM
2N
1N
AA6011
AA6011_3.5% NaCl_5mV
(Ovl) AA6011_1N_3.5% NaCl_5mV_1
(Ovl) AA6011_2N_3.5% NaCl_5mV
(Ovl) AA6011_Si/HMMM_3.5% NaCl
1
2
0.00000001 0.000001 0.0001
Current (A)
Potential (V)
0.01 1
13.19 Potentiodynamic polarization curves (E vs log I) of bare and
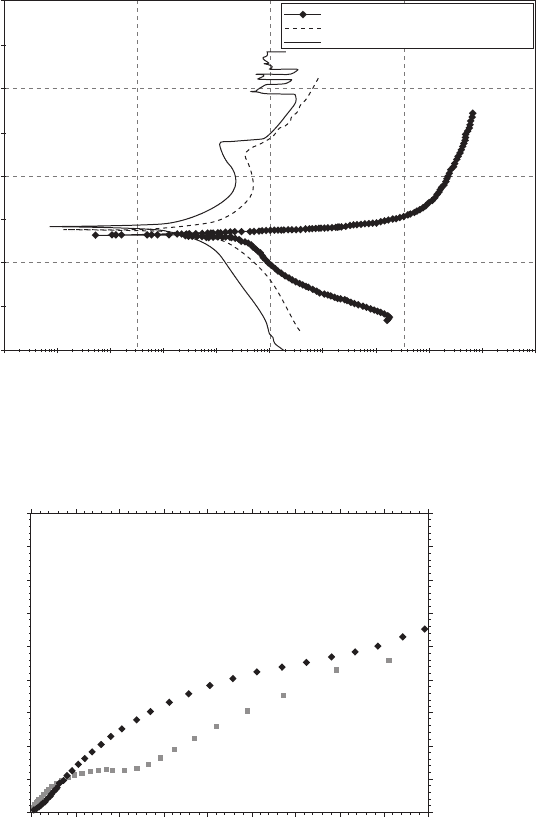
coated AA6011 in 3.5% NaCl at scan rate of 5 mV/s.
further improved the rate of film formation as well as the corrosion resis-
A, confirm the positive role
is limited since they are costly and difficult to apply. In recent years, cost
© 2008, Woodhead Publishing Limited
274 High-performance organic coatings
Along with their corrosion resistance, waterborne coatings also impart
improved hardness and hydrophobicity and enhanced UV resistance, as
discussed below.
–2
–1
0
AA6011_3.5% NaCl_5mV
(Ovl) AA6011_1N_3.5% NaCl_5mV_1
(Ovl) AA6011_1N/PTSA_3.5% NaCl
1
2
0.00000001 0.000001 0.0001
Current (A)
Potential (V)
0.01 1
13.20 Potentiodynamic polarization curves (E vs log I) of bare, Si/1N
and Si/1N-PTSA coated AA6011 in 3.5% NaCl at scan rate of 5 mV/s.
0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.5
4.0
450035002500
Bare AI
Coated AI
2000
Z′ (ohm)
–Z″ (kΩ)
15005000 1000 40003000
13.21 Nyquist plot of bare and coated (conventional polyester–MTMS–
GPTMS) aluminium in 3.5% NaCl at scan rate of 5 mV/s.
aluminium confirm the superiority of waterborne coatings (Fig. 13.21).
© 2008, Woodhead Publishing Limited
Waterborne coatings for corrosion protection 275
13.11.2 Mechanical properties
Coatings, generally, are subjected to stresses when fabricated into products
by rolling, bending, or other deformation processes. These stresses can
ture of the coating, exposing the substrate, scratch, or loss of adhesion to
the substrate. Therefore, to withstand the stress of fabrication, coatings
must adhere to the substrates on which they are applied, and be hard
enough to resist scratching during fabrication and during service life. A
variety of recognized methods can be used to determine how well a coating
is bonded to the substrate. Commonly used measuring techniques are the
cross-hatch test, the bend test, the pencil hardness test and the impact
test.
Bend and impact tests suggested that all MTMS–GPTMS coatings were
well adhered to the surface and were able to withstand the stresses during
service. The pencil hardness of MTMS–GPTMS coatings was found to be
above 6H. The hardness of 2N crosslinked coating was found to be better
than that of 1N crosslinked coating. Incorporation of organic polymers to
sol provides a coating of intermediate hardness between pure MTMS–
GPTMS coating and pure organic polymer-derived coating. However, the
hardness can be controlled by the MTMS–GPTMS and organic polymer
MTMS–GPTMS coating decreases with increasing polyester/alkyd content.
Figure 13.22 shows that the addition of conventional polyester in MTMS–
GPTMS sol reduces the hardness of the coating from 6H to 3H, depending
upon the polyester–sol weight ratios.
13.11.3 Hydrophobicity
The water contact angle is a quantitative measure of the wetting of the
coated substrate by water. The total surface free energy of a sol-gel coating
and its components can also be determined using Young’s equation, which
relates the surface tension of water in equilibrium with its vapour, with the
contact angle of the water drop on the surface of a sol-gel coating:
γ
SL
= γ
SV
− γ
LV
cosθ
where
γ
SL
= interfacial free energy between the bare/sol-gel coated substrate and
the water
γ
SV
= surface free energy of the bare/sol-gel coated Al in equilibrium with
water vapour
γ
LV
= surface tension of the water in equilibrium with its own vapour
θ = contact angle.
ratios. We observed that the hardness of the polyester and alkyd modified
exceed the flexibility or adhesive strength of the coating, resulting in frac-
© 2008, Woodhead Publishing Limited
