Khanna A.S. (Ed.) High-Performance Organic Coatings: Selection, application and evaluation
Подождите немного. Документ загружается.

166 High-performance organic coatings
range, making the polyesters a diverse group for coating applications.
Synthesis of aliphatic polyesters by polycondensation of diols with dicar-
boxylic acids traces back to Carothers’ work in the 1930s. However, the low
in obtaining high molecular weight polymers, had prevented wide usage of
aliphatic polyesters as polymeric materials for a long time [2, 3]. Later on,
with the advent of new monomers and synthetic protocols, the synthesis of
tailor-made polyester resins and their application span in coatings
increased.
9.3 Raw materials and reactions
The structure of the polyester can be varied by the ratio of di-, tri- and
tetra-functional monomers and degree of conversion. The polyester is
either acid-functional or hydroxy-functional depending on the mole ratio
of the ingredients used. The composition of the di/tri-functional monomers
can be varied perpetually, allowing the structure and properties of the
resulting polyesters to span over a very broad range [1, 4]. Diacids and diols
used for the preparation of polyester resins are far too plentiful to be men-
tioned here. Nevertheless, some structurally relatively simple diacids, diols,
tri- and tetra-functional monomers widely used in the coatings industry are
shown in Fig. 9.2.
Some of the most common glycols or polyols used for polyester prepara-
tion are ethylene glycol, 1,3-propanediol, 1,3-butanediol, 1,4-butanediol,
1,2-propylene glycol, 1,6-hexanediol, 1,5-hexylene glycol, isosorbide, ester-
diol, styrene glycol, bisphenol A, oligo(ethylene glycol)s, neopentyl glycol
OHHO
COOH
OO C
O
O
OH
O
O
n
+
n(diol)
n(diacid)
ester group
polyester
n(H
2
O)
COOH
COOH
+
HO
OH
H
2
C
H
2
C O
O O
O
n
terephthalic acid
ethylene glycol
polyethylene terephthalate
Example
9.1 Synthesis of polyester resin.
melting points inherent to aliphatic polyesters, together with the difficulty
© 2008, Woodhead Publishing Limited
Polyester coatings for corrosion protection 167
HO
O
O
OH
esterdiol (ED)
HO
OH
HO
trimethylol propane (TMP)
OH
OHHO
glycerol
OH
HO
1,4-cyclohexanedimethylol (CHDM)
HO
H
2
C
OH
n
n = 2 ethylene glycol
= 3 1,3-propanediol
= 4 1,4-butanediol
= 5 1,5-pentanediol
= 6 1,6-hexanediol
1,2-propanediol
OH
OH
OH
OH
OH
OH
OH
OH
2,2-diethyl-
1,3-propanediol
2-butyl-2-ethyl-
1,3-propanediol
2,2-dimethyl-
1,3-propanediol
(NPG)
OH
HO
2-methyl-
1,3-propanediol
OH
HO
HO
OH
pentaerythritol
O
O
OH
HO
isosorbide
H
2
C
n
n = 1 malonic acid
= 2 succinic acid
= 3 glutaric acid
= 4 adipic acid
= 5 pimelic acid
= 6 suberic acid
HO
O
OH
O
COOH
COOH
COOH
COOH
COOH
COOH
phthalic acid
isophthalic acid
terephthalic acid
OH
HO
O
OH
HO
O
OH
O
malic acid
NH
2
O
aspartic acid
COOH
COOH
COOH
COOH
1,3-cyclohexanedi-
carboxylic acid (1,3 CHDA)
1,4-cyclohexanedi-
carboxylic acid (1,4 CHDA)
phthalic anhydride
O
O
O
hexahydrophthalic
anhydride (HHPA)
O
O
O
trimellitic anhydride
O
O
O
HOOC
9.2 Different di-, tri- and tetra-functional monomers widely used in
the preparation of polyester resin for coating applications [1].
© 2008, Woodhead Publishing Limited
168 High-performance organic coatings
(NPG), trimethylol propane (TMP), di-trimethylol propane (diTMP),
mono-pentaerythritol (MPE), di-pentaerythritol (diPE), glycerol, 1,4-
cyclohexanedimethanol (CHDM), etc. Widely used diacids are malonic
acid, succinic acid, glutaric acid, adipic acid, pimetic acid, suberic acid,
azelaic acid, sebacic acid, isophthalic acid (IPA), phthalic acid, terephthalic
acid (TPA), fumaric acid, oxalic acid, aspartic acid, malic acid, etc. Trimellitic
anhydride, phthalic anhydride, hexahydrophthalic anhydride (HHPA),
etc., as dianhydride are widely used to prepare polyester coatings.
The most straightforward route to improve mechanical properties and to
minimize hydrolytic instability is the introduction of aromatic units into the
polyester main chain. The aromatic diacid compounds are used to increase
the glass transition temperature (T
g
), hardness, and chemical resistance.
This is why TPA is such a tremendously important building block in com-
mercial thermoplastic polyesters. In conventional systems, TPA usually
g
replacement of TPA by IPA, an isomer of TPA in the polyester backbone,
aromatic monomers have the disadvantage that they are more susceptible
to photo-oxidation, leading to yellowing of the coating with time [6–8]. In
the early 1990s, cyclohexyl dibasic acids were proposed as replacements for
the aromatic dibasic acids. The cyclohexane isomeric diacid monomers
which are typically used in the preparation of polyesters are hexahydroph-
thalic anhydride (HHPA), 1,3-cyclohexanedicarboxylic acid (1,3-CHDA),
and 1,4-cyclohexanedicarboxylic acid (1,4-CHDA). The cycloaliphatic
structure gives the intermediate physical properties between that of aro-
matic and linear aliphatic polyesters except the yellowing resistance, which
is better with linear diacids. Relative to aromatic polyesters, T
g
of cycloali-
phatic polyester is lower, but is higher compared to linear aliphatic polyes-
the aromatic and linear aliphatic polyesters due to the presence of cyclic
structure in the monomer that can absorb energy through the interconver-
sion of chair and boat conformations [9, 10].
9.3.1 Reaction setup
Usually two processes are used in the preparation of saturated polyesters,
i.e., the melt process and the solvent process. In the solvent process, an
azeotropic solvent such as toluene or xylene is used to take out the water
formed during the polycondensation reaction. The basic raw materials are
charged in the reactor and reaction is carried out in general at around 200–
240°C. The reaction is monitored by following the acid value periodically
[11] and stops when the acid value comes down below 5. A laboratory-scale
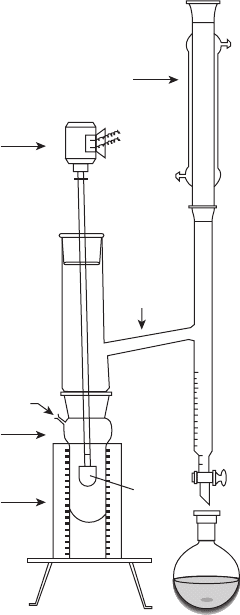
reaction setup for polyester preparation is shown in Fig. 9.3.
provides chain rigidity and thus sufficiently high T . On the other hand,
significantly increases the weathering resistance of coatings [5]. However,
ters. The flexibility of cycloaliphatic polyester is also intermediate between
© 2008, Woodhead Publishing Limited
Polyester coatings for corrosion protection 169
9.4 Polymerization methods
polyols. The mixture of monomers should be charged to the reaction kettle
and the reaction takes place after melting of the reactants with the elimina-
tion of water molecules (Fig. 9.4). When dianhydrides are used instead of
diacids, no water removal takes place from the system.
di-/polyols. During this reaction, methanol or ethanol (depending on the
acid ester used) is generally collected into a graduated receiver as a
Condenser
Stirrer motor
N
2
inlet
Glass reactor
Heating
mantle
Dean Stark
apparatus
Stirrer
blade
9.3 Laboratory-scale reaction setup for polyester preparation [12].
9.4.1 Direct esterification
The monomers for the polyesterification reaction are di-/polyacids and di-/
9.4.2 Transesterification
In transesterification, the methyl or ethyl ester of the diacid is reacted with
© 2008, Woodhead Publishing Limited
170 High-performance organic coatings
by-product to allow estimation of the extent of conversion. The electro-
shown in Fig. 9.5 [13, 14].
9.4.3 Effect of catalysts
Many researchers have studied the effects of various catalysts for both the
catalysts such as dibutyltin oxide, dibutyltin dilaurate, titanium tetrabutox-
ide, titanium (IV) n-butoxide, p-toluene sulfonic acid (p-TSA), etc., are
based catalyst is more active than antimony- and tin-based catalysts. The
activity of the polycondensation catalysts follows the order Ti > Sn > Sb >
Mn > Pb [14]. However, titanium-based catalysts have the disadvantage of
imparting a yellow color to the polyester [15].
9.5 Determination of hydroxyl value and acid value
hydroxide (KOH) equivalent to the hydroxyl content of one gram of the
sample. Hydroxyl value is estimated by following the method set out in
grams of potassium hydroxide required to neutralize the free carboxylic
acid present in one gram of the polyester. The acid value of the resin is
R OH
O
+
+
R O
O
R O
O
O
HO
R
1
OH
R
1
HO
R O
O
R
1
OH
(+)
H
H
(+)
Transition state
()
H
H
H
+
H
2
O
R OR
1
O
+
cat
+
R OR
1
O
cat
R OR
1
O
cat
O
HO
R
2
OH
R
2
HO
R O
O
OR
1
R
2
OH
+
cat
+
δ
+
δ
+
9.4 Mechanism of direct esterification reaction.
9.5 Mechanism of transesterification reaction.
philic mechanism for metallic catalysis of the transesterification reaction is
transesterification and polycondensation reactions. Tin-based esterification
widely used as esterification catalyst. Different results show that titanium-
The hydroxyl value is defined as the number of milligrams of potassium
the ASTM standard [16]. The acid value is defined as the number of milli-
© 2008, Woodhead Publishing Limited

Polyester coatings for corrosion protection 171
estimated as in the ASTM D 1639–70 method [17] by titration of the sample
with standardized KOH or NaOH solution. The solvent in the sample must
be removed and the sample in solid basis must be weighed. Then the sample
is dissolved in isopropyl alcohol and toluene. Phenolphthalein is used as
indicator and a pink coloration indicates the end point. The acid number
is calculated from the following equation:
Acid number
KOH KOH
sample
=
××VNMw
m
KOH
where V is the volume of potassium hydroxide solution consumed in mL,
N is the normality of the solution, and m is the weight of the sample in
grams (solid basis).
9.5.1 Effect of hydroxyl and acid number
In general, the synthesized hydroxyl or carboxyl terminated polyester resins
are crosslinked with different crosslinkers in thermoset coatings to obtain
desired properties. Therefore an increase in hydroxyl or acid number in the
polyester backbone results in an increase in the crosslinking density of the
high solid coatings
In general an increase in the hydroxyl number of polyester resin produces
thermoset coatings with better chemical resistance and mechanical proper-
ties. This is due to the increase in the crosslink density of the coatings.
However, an increase in the hydroxyl number in the resin produces increased
intermolecular hydrogen bonding, which increases the viscosity of the
system. Therefore it is essential to modify the resin structure to give low
viscosity and good crosslink density with the crosslinker. This can be
achieved by partial replacement of the available hydroxyl groups in hydroxy-
lated polyester with the less polar acetoacetate groups, which leads to an
increase in solid content at the application viscosity as well as increase in
adhesion due to the chelate effect (Fig. 9.6). Acetoacetylated or β-ketoester
incorporated polymers offer a versatile crosslinking mechanism. This ver-
satility results from the presence of two sites available for crosslinking
reactions. These sites are the active methylene group and the ketone
carbonyl group. β-ketoester groups are amphoteric and can participate in
a variety of chemical transformations, which might be used to modify or
crosslink polymers. There are several routes by which acetoacetylated
ferred for coating applications [18–21]. The crosslinking reaction of the
final thermoset coatings.
9.6 Backbone modification of polyester resin for
materials can be prepared, of which the transesterification route is pre-
© 2008, Woodhead Publishing Limited
172 High-performance organic coatings
active methylene group in acetoacetylated polyols with diisocyanate pro-
duces additional crosslink density with superior properties and weathering
stability [22–25].
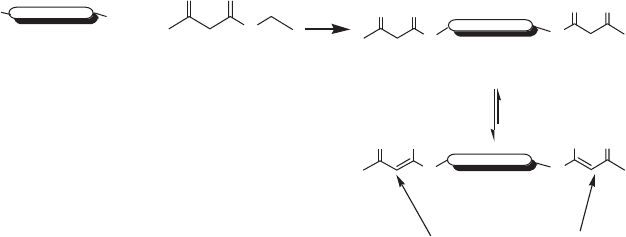
9.6.1 Functional branched polyesters for
coating application
The ability to synthesize new polymers with predetermined and controlled
structures is important when new materials are to be created with desired
physical and chemical properties. Hyperbranched polymers (HBPs) have
been extensively studied as part of recent research efforts aiming at tailor-
ing the properties of polymeric materials by variation in the molecular
architecture. HBPs are highly branched structures based on AB
x
or A
x
B
monomers (x ≥ 2 and A and B are hydroxyl and carboxylic acid moieties,
respectively), which introduces potential branching points in every repeat-
ing unit as well as at the end-groups. The large number of end-groups and
branching points gives HBPs different properties compared to their linear
analogs. For instance, HBPs have low melt and solution viscosity and high
solubility, and are easy to modify to obtain tailored properties [26]. The
lower solution viscosity of HBPs compared with linear polymers is due to
the lack of entanglements, which result from their packed structure. They
also often possess very high solubility compared to those of linear polymers,
as a result of the large number of peripheral terminal functional groups
available per macromolecule. Therefore, the use of HB polyesters as spe-
sizing new three-dimensional networks with controlled architecture [27].
Recently, different methodologies for constructing HB polyester polyols
from AB
x
monomers have been explored. The routes involve thermally
driven homo-polycondensation or activation of either A or B functional-
O
O O
O
O O
O
O O
+
HO
OH
keto form
O
O OH
O
OH O
enol form
active methylene group
ethyl acetoacetate
- a β keto ester
hydroxylated polyester
1,4-addition
addition reaction.
9.6 Backbone modification of hydroxylated polyester by Michael
cific crosslinking agents for coatings seems a very promising way of synthe-
© 2008, Woodhead Publishing Limited
Polyester coatings for corrosion protection 173
ities. Thermally driven polycondensation of AB
2
monomers uses p-TSA or
an organometallic catalyst. Huybrechts and Dusek [28] reported that the
properties of low volatile organic component (VOC) coating formulations
containing HB polyol are superior to those of linear polyols. In Sweden, a
systematic investigation of HB polyesters as curing agents was developed
[29, 30]. At present, several dendritic/HB polyester polyols are commer-
cially available from Perstorp Polyols, Inc., Perstorp AB, Sweden, with the
trade name ‘Boltorn’, and are easy to synthesize [31]. For instance, HB ali-
phatic polyester prepared from 2, 2-bis(methylol) propionic acid (DMPA)
and 2-ethyl-2-(hydroxymethyl) 1,3-propanediol was used as crosslinking
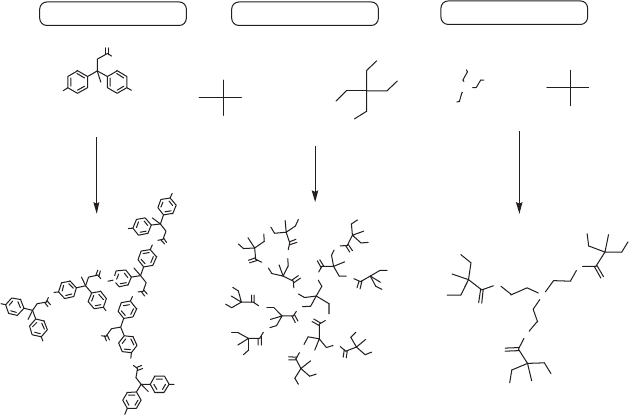
agent in coating formulation. Ziemer et al. [32] synthesized HBP using 4,4-
bis(4-hydroxyphenyl) valeric acid as an A
2
B type raw material (Fig. 9.7(a)).
Researchers also proposed the polycondensation reaction of AB
2
monomer
in the presence of a B
n
core molecule as an improved method to control the
condensation reaction [33]. Examples of such an AB
2
-monomer/B
n
-core
approach include the preparation of HBPs from DMPA (an A
2
B monomer)
and pentaerythritol [34] (Fig. 9.7(b)), triethanol amine (Fig. 9.7(c)) and
trimethylolpropane (TMP) [35, 36] as core molecules. Pavlova et al. [37]
used HB polyester prepared from 4,4-bis-(4′-hydroxyphenyl)pentanoic acid
and TMP as core molecule for coatings preparation. The commercially
available (Perstorp Polyols, Inc.) hydroxyl-functional HB polyester of
the third generation (G3) prepared from DMPA and ethoxylated
COOH
CH
2
OH
CH
2
OH
HO
OH
OH
HO
O
O
O
O
O
O
O
O
O
O
O
O
OH
OH
OH
O
O
O
O
OH
OH
O
O
O
O
OH
HO
HO
HO
OH
OH
O
O
O
O
HO
HO
HO
HO
OH
Boltorn
TM
H20
pentaerythritol
DMPA
p-TSA catalyst
N
HO
OH
HO
p-TSA catalyst
N
O
O
O
O
HO
HO
O
HO
OH
O
HO
OH
HO OH
OH
O
OH
HO
O
O
O
O
O
O
O
OH
O
HO
O
HO
OH
O
O
HO
OH
4,4-bis (4-
hydroxyphenyl) valeric
acid
DBTL catalyst
A
2
B approach
COOH
CH
2
OH
CH
2
OH
A
2
B + a core molecule
A
2
B + a core molecule
(a)
(b)
(c)
+
+
DMPA
triethanolamine
9.7 Different synthetic approaches for the preparation of hyper-
branched polyols [59].
© 2008, Woodhead Publishing Limited
174 High-performance organic coatings
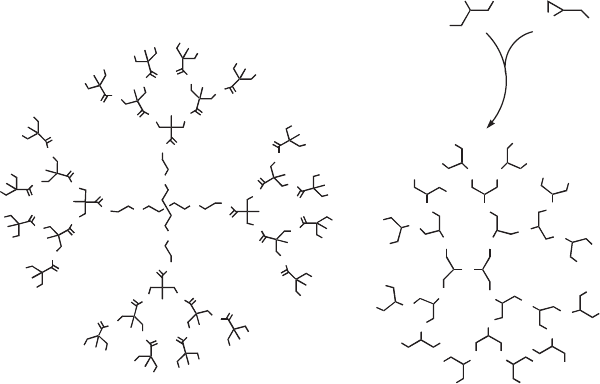
pentaerythritol (a core molecule) is shown in Fig. 9.8(a). An A
2
+ B
3
approach produces an AB
x
molecule as an intermediate during the conden-
sation reaction and is important due to the commercial availability of A
2
and B
3
type monomers. As an example, the synthesis of aliphatic HBP
polyol from adipic acid and glycerol can be mentioned [38]. Recently, Frey
and coworkers [39, 40] and Xinling and coworkers [41, 42] developed diffe-
rent synthetic routes that emerge from the combination of the multi-branch-
ing polymerization of glycidol with well-established epoxide polymerization
techniques, leading to unprecedented polymer architectures. The synthesis
of hyperbranched polyglycidol (HPG) involves the cationic ring-opening
diethyl etherate catalyst in chloroform. The reaction was carried out at 25°C
for more than 3 h and in a nitrogen atmosphere (Fig. 9.8(b)).
9.7 Polyester-melamine coatings
Melamine-formaldehyde (MF) resins are important components of ther-
mosetting resin products. They are widely used in the coatings industry as
binders and crosslinkers to produce highly durable coatings. Thermoset
coatings based on the reaction of MF resins with hydroxyl functional
O
O
O
OO
O
O
O
O
O
O
O
O
O
OH
OH
OH
OH
O
O
O
OH
OH
O
OH
OH
OO
O
O
O
O
O
O
O
O
HO
HO
O
O
O
OH
OH
O
OH
OH
OO
O
O
O
O
O
O
O
O
OH
OH
OH
OH
O
O
O
HO
HO
O
HO
HO
O
O
O
O
O
O
O
O
O
O
HO
HO
HO
HO
O
O
O
HO
HO
O
HO
HO
HO
HO
OHHO
HO
O
OH
BF
3
O(C
2
H
5
)
2
O
O
O
OO
O
O
OH
OH
O
OH
O
OO
OH
HO
OHHO
HO
O
O
OHHO
HO
HO
O
HO
O
OH
O
O
O
OH
OH
O
OH
OH
OO
OHHO
HO
OH
HO
OH
Hyperbranched polyglycidol
base catalyst or
Hydroxy functional HBP of the third generation (G3)
(a)
(b)
9.8 A third-generation hyperbranched polyol (a) and hyperbranched
polyglycidol (b).
polymerization of glycidol, by glycerol in the presence of boron trifluoride
© 2008, Woodhead Publishing Limited
Polyester coatings for corrosion protection 175
polyester have long been used in the coatings industry. For instance, poly-
ester resin crosslinked with hexamethoxymethyl melamine (HMMM) were
developed in 1972. MF crosslinkers have been shown to give improved
chemical, heat, wear and scratch resistance, improved hardness, and
exterior durability to coatings. Typical applications include coatings use in
household appliances, automotive OEM coatings, industrial coatings for
coils and cans, agricultural and construction, etc. Because of their value,
MF resins have been extensively studied in order to optimize the properties
temperatures above 100°C for 10 to 40 min in the presence of p-TSA cata-
lyst. The wide acceptance of p-TSA is due to the compatibility of its molec-
ular structure and hydrophobic nature with the paint components.
9.7.1 Melamine-formaldehyde resins
MF resins are mixtures of compounds with different degrees of substitu-
temperature, pH of the medium, reactant ratio, and the degree of polym-
erization. HMMM resins are the reaction products of melamine (2,4,6-
triamino-1,3,5-triazine) with formaldehyde to form methylol groups and
converting these methylols to methoxymethyl groups using methanol. Up
to six moles of formaldehyde can be combined with one mole of melamine
to produce hexamethylol melamine. In the preparation of coating resins,
usually 5–6 moles of formaldehyde are reacted with one mole of melamine.
The degrees of methylation and methoxy-methylation are varied according
to the intended end-use applications. HMMM resin may contain, besides
pure HMMM, also dimers and trimers, and other end-groups than
2 3
nism and rate of reaction. Furthermore, during the crosslinking reaction
self-condensation of HMMM can occur between the various functional
groups. HMMA is a versatile crosslinking agent for a wide range of poly-
meric materials, both for solvent-borne high solids and for water-borne
coatings. Butylated melamine resins (BMF) are widely used in industrial
cured coatings. They are widely used for interior applications, interior
9.9 shows the structure of fully substituted methylated MF and butylated
MF resin.
9.7.2 Reaction of polyester with melamine resin
Hydroxylated polyester resins are generally crosslinked with BMF and
HMMM in a ratio of 90/10 to 70/30. The curing reaction (Fig. 9.10) is
catalyzed by a strong acid such as p-TSA. The catalyzing mechanism as
of the final coating. Such formulations are commonly cured by baking at
tions [43]. The final form of the MF resin produced depends on the reaction
stoving primers and enamels, in acid-catalysed wood finishes and in force-
container coatings, and two-component solvent-based wood finishes. Figure
–CH OCH . The various functional groups influence the reaction mecha-
© 2008, Woodhead Publishing Limited
