FTA (изд-во). Flexography: Principles And Practices. Vol.1-6
Подождите немного. Документ загружается.

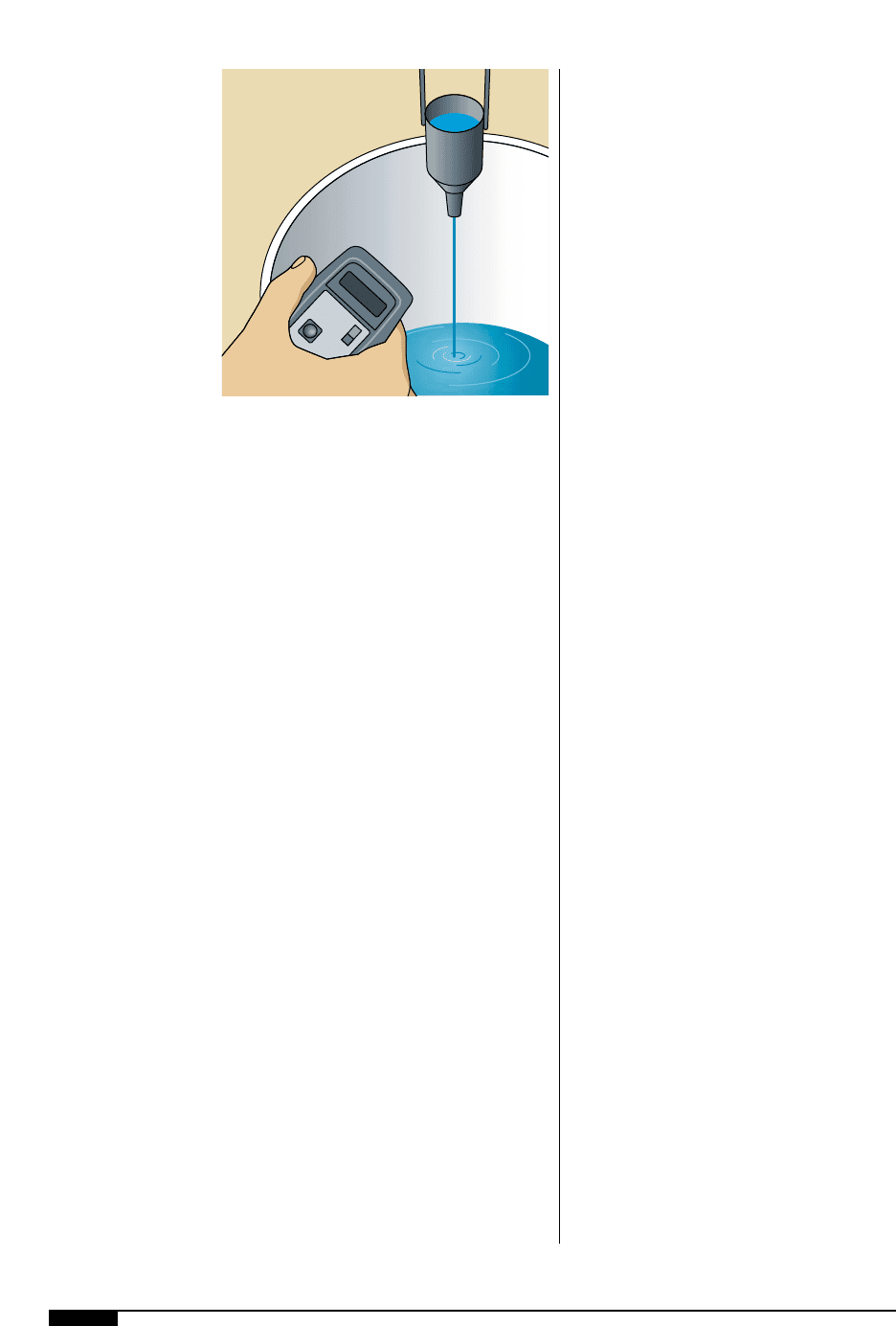
92 FLEXOGRAPHY: PRINCIPLES & PRACTICES
Regardless of which instrument is used to
measure viscosity, calibration must be done
on a regular basis. Slight variations in read-
ings can result in significant print problems.
Calibration should be done when new equip-
ment is received, and a log should be estab-
lished to maintain a regular calibration pro-
gram.
Color Adjustment at Press
The basics of ink viscosity – how it is mea-
sured and some of the factors that affect it
have been discussed. The next topic is how
to use ink viscosity and ink metering to
adjust and control color strength. In any
printing configuration, the actual metering
system will determine the ink strength need-
ed to achieve the desired color. The color
strength achieved when ink is added to the
press may be acceptable. If it is too strong,
however, at least three options exist:
• Add solvent or water depending on the
system, thereby reducing viscosity and
strength. This is the easiest way to
adjust down color strength. It may,
however, lead to drying problems in
water-based inks or even drying prob-
lems in solvent systems.
• Add extender varnish; this second way
to reduce color strength is often best as
it provides color strength reduction
with the least effect on drying or block-
ing and optimum color value.
• Change the anilox to reduce color vol-
ume. This approach provides the least
effect on the ink itself and probably the
best long-term balance of good printing
and high color intensity.
On the other hand, if the ink is too weak,
different options exist:
• If the ink is pre-reduced on press start-
up, uncut ink should be added to the
fountain or reservoir. This will build
strength and viscosity. This is certainly
the easiest cure if the higher viscosity
has not caused printing quality prob-
lems. Increasing viscosity and increas-
ing strength often go together with dirty
printing.
• If an ink based on a dispersion and let-
down varnish is being used, additional
dispersion may be added. This can
eventually lead, however, to poor adhe-
sion or a loss of other properties as the
dispersion is not a complete ink vehicle.
• If other factors are equal, changing the
anilox to a higher volume is certainly
preferred. Here more ink is carried to
the substrate at the lowest viscosity pos-
sible to provide optimum strength with
the cleanest colors.
Printing the same color ink at excessive
strength or viscosity makes the color itself
muddy looking. At the same time, dirty print-
ing often occurs. A more transparent, finer
dispersion will negate this effect somewhat.
However, it often does so with a loss of color
intensity and an increase in cost. Sometimes
black is added to an ink to “fake” higher
strength. While this trick often will help, it
does so at the expense of color sharpness,
and the result is somewhat muddy.
Ideally, the ink supplier will provide an ink
that will yield the proper color values and
intensities with only minor metering or vis-
7^
Another method of
checking viscosity is
using a Shell Cup,
which is more
commonly used in
gravure applications
than in flexo. However,
because it is more
accurate than a Zahn,
printers favor this
method for on-press
checks.
M
IN
S
E
C
/1
0
0
7^

cosity adjustments. If, however, the ink is
already on press and color changes are nec-
essary, there are several areas of concern:
• A good, well-lighted area is needed for
color viewing. A standard light box is
recommended, since color varies under
different lights. A well-lighted area in the
pressroom is the last chance to ensure a
good color match to a color standard. A
board of retained prints from previous
rolls is also important for comparison so
that no color drift occurs. Whatever
standard light is used, it should be com-
parable to the light that the product will
be viewed by.
• Metamerism is defined as two colors
that match under one light source, but
are different under another light source.
A standard light source in the press-
room will help prevent this effect.
However, metamerism is not due only to
light effects on the color; different pig-
ments used in a color match will also
create the effect. Therefore, if matching
at press-side, try to use the same pig-
ments as originally used in the color
match. If this is not possible, be alert to
metamerism problems that may occur.
If metamerism does occur, the only solu-
tion is to change pigments.
• If color is altered at press side, be sure
that end-use problems aren’t created. All
colors have different fastness proper-
ties, and the end use for a given print job
may have very specific needs.
• The substrate is also of great concern. If
it has a color of its own, this may well
affect the outcome of the print job. It
may be necessary to use opaque pig-
ments to hide the substrate. These
opaque pigments will also, of course,
have an effect on other colors on the
job. If inks are used to hide the sub-
strate, metering effects and viscosity
control become critical. Any changes
during the run will create color shifts.
• Finally, another area of concern with on-
press color adjusting is the combination
of ink trap and ink transparency. If the
job being printed has multiple traps
(whether these be color-over-white or
color-over-color), the final color
achieved is a combination of the colors
involved. Any changes in the color
matches of the involved single colors
will alter the trapped colors as well.
These changes may involve revising a
particular color match on press or alter-
ing the transparency of the ink involved
with the actual color staying the same.
Both of these changes can affect the
final color printed.
Trapping. The trapping of one color over
another is very dependent on ink metering,
viscosity and drying. All of these factors
must be at their optimum for good trapping.
First-down inks that dry too slow or are
too low in viscosity will allow second-down
inks to dive into them, creating muddy col-
ors and dirty print. For good trapping, first-
down inks must dry faster than subsequent
inks. Thinner inks usually dry faster, but in
water systems this may not be true because
there is more water to dry. Excessively low
viscosity can cause dirty, sloppy trapping. A
balance has to be maintained as high viscos-
ity and fast dry can cause dirty colors in the
trap, as well as poor color fidelity.
MANAGING pH WITH
WATER-SOLUBLE INK SYSTEMS
Perhaps the most essential element of
water-soluble inks on press is the proper
control of pH. This is not an issue for sol-
vent-based or UV inks. Water-soluble inks,
however, can become virtually useless if the
pH is not maintained correctly.
What Is pH?
The pH value is the degree of acidity or
INK 93

94 FLEXOGRAPHY: PRINCIPLES & PRACTICES
alkalinity of a substance measured on a scale
of 0 to 14. From 0 to 7 is acid, and from 7 to
14 is alkaline. The neutral point is 7. Although
many believe water to be neutral, it is impor-
tant to remember that water is usually, but
not necessarily, approximately 7 pH. The pH
of water is determined by the pH of the soil
in the surrounding source area. In some
areas, this pH is also affected by the pH of the
rain water (acid or otherwise).
Resins used in the manufacture of water-
based inks are both of the solution and emul-
sion types, which can be carefully formulat-
ed for tailor-made performance relative to
specific press speeds, drying conditions,
application volumes and the like. The resins
used are generally alkali-soluble, acrylic
polymers. Simply, that means the resins –
when synthesized into high molecular
weight polymers – have numerous active
acid sites. In this slightly acidic condition,
the resins are not suitable for printing and
are coiled. The result is that the body of the
polymers is heavy, and the viscosity is very
high, rendering ink neither pourable nor
pumpable in a press-and-ink pan loop. When
the polymers are adjusted with an amine or
other alkali to an alkaline pH range of 8.0 to
9.5, the resins perform optimally and have
the best characteristics for dispersing and
wetting-out pigments, for transferring and
laying out on the substrate, and imparting
the product resistance requirements.
While printing with water-based inks, the
heat produced from running the press and
heat from the outside environment can lower
the pH of the ink by evaporating the ammo-
nia and/or amines in the ink. As the amine
evaporates, the pH of the ink falls, and the
resin begins to revert back to the heavy-body,
higher viscosity ink. At that point, adjusting
the viscosity with water will not quickly or
effectively lower the viscosity or heavy body
because it is a chemical problem and not a
physical one. It is very important that the pH
of the ink be raised. One 6-ounce cup of
ammonia or an appropriate amine aded to 20
gallons of ink will raise the pH of the ink
from 8.0 to 8.9. A heavy, high viscosity ink
can occur at a pH around 8.1 to 8.3. Table 13
outlines what happens with an ink and pH.
In summer months, it is advisable to mon-
itor and adjust pH on an hourly schedule. In
the cooler months, every two to three hours
should be adequate.
When the pH of an ink becomes too low,
the ink will begin to body up or get higher in
viscosity. The resins in the ink begin to fall out
of solution. The lids on drums or buckets of
ink can sometimes be seen to have ink strings
coming from them as a result of poor resin
solubility. At low pH, the ink will also begin to
transfer improperly from the anilox to the
plate and from the plate to the substrate,
causing a decrease in color strength. Also, the
inks will start to build up on the plates, caus-
ing dirty printing. These are some of the
noticeable signs of low pH. Other problems
could occur with the printed material as well,
which are harder to detect. If the pH of the
ink is too high, the printed material will usu-
ally have poorer water resistance than nor-
mal. This may not be an issue if the ink is not
designed for water resistance in the first
place. The job may be run without any notice-
able print problems, but there is a chance for
a potential claim when the print rubs off
under wet conditions. In addition, if the inks
are too high in pH, the amine odor can also
become a problem.
How pH is Measured
There are several different ways to mea-
sure pH. The best way is to use a reliable pH
meter, which can be purchased from any
scientific equipment facility .and can cost
from $100 to several thousand dollars. For
the purpose of ink-pH control in the press-
room, a pH meter costing several hundred
dollars is usually sufficient.
The least-expensive version is a pocket
model, which has too high a variance range

for these purposes. These only read to one
decimal place and have a variance of ±0.2.
With this type of instrument, a pH reading of
8.5 might appear to be up to specification.
However, with a variance range of ±0.2 the
ink could actually have a pH of 8.3, which is
too low and could create printing problems.
A pH meter which reads to two decimal
places and has a variance range of only ±0.01
is recommended. Using this type of meter,
the same ink that reads between 8.3 and 8.7
would show a pH of 8.49 ±0.01.
This doesn’t mean that the pocket model
doesn’t have its place in the pressroom. For
example, it is feasible to have a pocket
model at every press, but impractical to have
a $200 model at every press. It is important
that press personnel be trained to check the
pH of water-based inks at least every four
hours. If they have a pocket model, which is
also much easier to use, they can check the
pH of the inks often and easily. If the ink has
a pH of 9.0, even with a ±0.2 variance, the ink
would still be in spec. If they run into situa-
tions where the pH of the ink is question-
able, a sample of the ink could be brought to
the location of the more accurate instru-
ment, usually the quality control laboratory.
If the only pH meter in the facility is the one
in the quality control laboratory, the likeli-
ness of press personnel bringing samples of
all the inks to be checked every four hours is
slim to none. Purchasing anything more than
an instrument which reads to two decimal
places and a variance range of ±0.01 is
overkill for many purposes. Some of the
more expensive units can read to four or
even six decimal places with variance ranges
of 0.000001. This type of accuracy would
never be needed with water-based inks.
Adjusting pH
When running press-return inks (inks
which have previously been used) or run-
ning for a long period of time, the pH of a
water-based ink can fall. When the pH of the
ink has slipped below its specified level, an
adjustment is usually necessary.
The ink supplier will specify how and with
what amine to adjust the pH. Different
water-based inks will need adjustments dif-
ferently. If a new water-based ink system is
being used in the pressroom, the first ques-
tions that should be asked are: How often
should the pH be checked? What amine
should be used to adjust the pH? How
should it be added? What is the proper pH
range for this ink system?
The proper way to adjust the pH of most
water-based inks is to make up a mixture of
water and ammonia, or whatever amine is
recommended. This mixture should be
approximately nine parts water to one part
amine. Undiluted amine should never be
added to the ink, because it can cause a
shocking effect to the ink, and make it kick
out, or have suspended particles form. Even
if the ink doesn’t kick out, it is very difficult
to adjust the pH properly with a full strength
amine. An ink that is at pH 8.0 requires only
a slight increase to reach the prper level. It
INK 95
Table 13
6.0–7.0 PRECIPITATE:
Ink and resin separate. Result is a
high viscosity and a poor print
7.0–8.0 INK UNSTABLE:
Dirty, fuzzy print; high viscosity,
heavy body and some buildup on
anilox and plate.
8.0–9.5 GOOD FLOW CHARACTERISTICS:
Good print, good adhesion and
excellent wet-out properties.
If viscosity is high, adjust by adding
2%–5% water. High viscosity does-
n’t necessarily mean a low pH, but a
low pH means a high viscosity.
9.5–11.0 POTENTIAL PIGMENT BURN-OUT:
Excess foam, corrosive to steel
and iron. Lack of water resistance is
possible.
PROPER pH CONDITIONS FOR
WATER-BASED INKS

96 FLEXOGRAPHY: PRINCIPLES & PRACTICES
would be very easy to add too much amine,
and the result could be an ink with a pH of
10, 11, or higher.
The alkaline mixture should be added
slowly while agitating the ink. The viscosity
of the ink should decrease as more alkaline
mixture is added. This is partly because of
the additional water, but more importantly,
the resultant lower viscosity is due to the pH
rising to the desired range. Add only a little
at a time, stopping to check the pH, making
sure not to overshoot the target range.
Unfortunately, there is nothing that can be
added to inks which are too high in pH. It
might seem that adding an acid would
reduce the pH level. In theory, it might give
the desired pH, but the resins and additives
in the inks are intolerant to acids and the
result would be wasted ink. The only possi-
ble addition to an ink with too high a pH
would be more virgin ink, and this is only
effective if the pH of the virgin ink is lower.
When adding amines, stop once the ink is in
the desired pH range. A pH level of 9.5 is not
better than 9.0; anywhere in the range is
ideal. Adding more amine once desired
range is achieved only increases the chances
of overshooting the range.
After the pH is in the desired range, the
viscosity should be checked to ensure it is
still where it needs to be. If the viscosity is
too high, a little plain water can be added to
get to the desired viscosity. Always adjust
the ph before adjusting the viscosity!
For almost any problem encountered
while running water-based inks the pH level
is the first thing to check. While pH is not the
only problem that will be encountered, and
pH is not the root of all water-based ink
problems, it is a good first step.
WATER- VS. SOLVENT-BASED INKS
Water dries more slowly than most sol-
vents. With many printers moving to water-
based inks, how do they deal with slower-
drying inks? Ironically, one of the largest
water-based problems we see is that the inks
dry too fast, and solvents need to be added
to remedy this problem. The following will
hopefully explain how this actually works.
Most water-based inks are formulated to
be stronger in color strength. Therefore, less
ink is needed to achieve the desired color
strength than solvent-based inks. By apply-
ing less ink, usually by using finer line
aniloxes, less water is also applied. The less
water applied, the less water there is to dry.
In addition, many printers run water-based
inks at slightly higher viscosities than sol-
vent-based inks. This allows for more color
strength with less water present in the ink.
Finally, resins unique to water-based sys-
tems are incorporated in the form of emul-
sions. The resin is not dissolved in the water
the way it is in a solution varnish but
remains suspended as a particulate. What
this does for drying is that it allows virgin
inks to be formulated at lower viscosities.
The lower the viscosity of a virgin ink, the
less water it takes to reduce it to press print-
ing viscosity. Once again, the less water, the
easier the drying.
With all these methods of reducing the
amount of water in the final ink film, a point
is reached where in many cases, slow sol-
vents (often glycol ethers) are added to
water-based inks to slow them down, so they
print cleaner. This has created problems for
many printers over the years, where the use
of these glycol ethers has sometimes
become rampant. Many press operators see
that a little glycol helps them to print clean-
er causing less downtime to clean plates,
and assume that if a little is good, more is
better. This assumption is wrong! Glycol
ethers need to be used only when absolutely
necessary and only in the recommended
amounts. Too much glycol ethers added to
inks will cause long-term problems that the
press operator won’t see.

First, glycol ethers do not always dry com-
pletely even with the best of dryers. The
retained glycol ether in the print can cause
blocking problems in the rewind. Sometimes
the retained glycol ether causes only slight
blocking, but when unwound from the roll,
the gloss of the ink is severely diminished.
Also, water resistance can be affected by
retained glycol ethers. Using glycol ethers can
be an asset to the pressroom, but only when
used correctly. A good general rule for the
addition of glycol is to add 2% to the sump
while agitating. If more is needed, add 1% at a
time until a maximum of 5% is reached.
Consult with your ink representative for
recommendations based on your ink system.
Drying speed of an ink is dependent on the
water and solvents in that ink, as well as on
the pH of the ink and the amine or ammonia
used in it. The most common amine used for
fast drying in water-based inks is ammonia,
but, many other amines exist and are widely
used due to drying speed considerations or
performance issues. Ammonia is used more
widely because its odor is considered less
offensive than many other amines, and it is
not a volatile organic compound (VOC). If an
ink is too high in pH, the smell of the amine
or ammonia can sometimes fill the press-
room causing eye irritations or even rashes.
Although this usually occurs only in severe
cases, many people have allergies to amines.
Care must be taken to avoid excess amine
and to keep press fountains well covered.
CLIMATIC EFFECTS
How well an ink performs on press is
affected by the pressroom conditions.
Temperature and humidity will affect how
well an ink will dry and therefore how fast a
press can be run. This is especially true for
water-borne inks. In addition, the pressroom
conditions can also affect resin solubility,
ink viscosity and overall ink performance.
Whether running water-borne or solvent-
based inks, climate is an important variable
that must be understood.
Humidity
Since water-borne inks dry by evaporation
of water from the print, conditions which
are optimal for this removal will assist in
drying. Dry air can accept more moisture
than humid air. Higher humidity can result in
poorer press speeds.
Solvent-based inks can also be affected by
climatic conditions. High humidity can
result in the alcohol in the ink absorbing
moisture from the air. This additional water
in the ink can cause certain resins to have
poorer solubility and result in dirty printing.
Keeping fountains covered and reducing the
amount of ink in the sump can minimize this
absorption of moisture by the ink.
Temperature
Warmer air can hold more moisture than
colder air. Therefore, water inks will dry best
under warm conditions with low humidity. To
a lesser degree, solvent inks are affected by
climatic temperature conditions. Solvent will
evaporate more quickly under higher temper-
atures. Due to the relative evaporation speeds
of common solvents used in flexo inks, the
window is much greater for acceptable tem-
perature conditions with solvent-based inks
then it is with water-based inks.
Temperature and humidity are closely
related to water-borne-ink performance. If
humidity is high, additional heat may be
required. If the temperature is high it may
increase the level of humidity that will result
in acceptable results.
Air Circulation
For water-based inks, dryers on the press
should also be designed to allow for maxi-
mum air passage. This will allow the saturat-
ed air to be replaced with lower humidity air
and assist in better drying. Care should be
used to prevent unbalanced dryers. Unbal-
INK 97

98 FLEXOGRAPHY: PRINCIPLES & PRACTICES
anced dryers, which blow air onto the plates
and/or anilox rollers, can result in ink drying
on the plates or drying in the anilox roller
causing dirty printing. Increasing tempera-
ture alone on a press running water-borne
inks may not be enough to attain acceptable
drying results. Optimum conditions for water-
borne inks are high temperature, low humidi-
ty and maximum air circulation.
Air circulation in the dryers is also impor-
tant for solvent-based inks. Since the air
coming into the ovens from the outside is
low in solvent content, it can readily accept
the solvents from the inks. With the move to
solvent incineration and solvent recovery,
dryers are being designed to permit higher
levels of solvent to accumulate before the air
is removed. This optimizes the incineration
or recovery aspect of the dryers. Under
these conditions, solvent removal from the
printed web may be lessened, due to the
higher solvent content in the dryers. Where
there is no solvent recovery or solvent incin-
eration, concern over solvent levels in the
dryers is not a major issue if the dryers are
performing correctly.
Climatic Effects on Ink Blocking
High humidity or high temperatures can
cause conditions in the rewind that will
result in ink blocking. High humidity can
cause condensation on the chill rollers, and
this moisture can transfer to the substrate.
Moisture in the rewind can cause certain
substrates to block. Chill rollers should be
checked on a regular basis to ensure they
are not forming condensation on the sur-
face. High humidity can also prevent com-
plete drying of water-borne inks. This resid-
ual moisture in the inks can increase poten-
tial blocking problems.
Blocking of the web is affected by temper-
ature, pressure and time. If high temperature
conditions persist, the printed web may not
be cooled properly before rewinding. This,
coupled with too high a rewind tension, can
promote blocking. To control this situation,
the use of chill rollers on the press is need-
ed. These chill rollers must be monitored to
spot condensation and correct the problem
immediately.
Climatic Effects on Ink Solubility
One of the most serious concerns with ink
solubility involves the absorption of water
into the alcohol in flexo inks. If these inks
also contain certain resins, such as nitrocel-
lulose, the presence of the water will signifi-
cantly affect the solubility of the nitrocellu-
lose resin. The result is a blushing of the ink,
giving it a flat appearance or causing a kick-
out. The solution usually involves switching
to a higher-molecular-weight alcohol, adding
additional acetate or adding glycol ether sol-
vents. Care must be taken not to add too
much acetate to avoid potential plate-swell
problems. If slower solvents are used, block-
ing may become a concern.
Water-based inks may show poorer solu-
bility in high heat conditions due to a loss of
amine in the ink. Most water-borne inks for-
mulated for printing nonporous substrates,
use a very fast-drying amine, which tends to
evaporate out of the ink under high temper-
atures. Loss of the amine results in poorer
resin solubility. By covering the sumps and
minimizing the amount of ink in each sump,
the evaporation of amine can be reduced.
Climatic Effects on Dirty Printing
Dirty printing can be the result of poor ink
resolubility. It can result from the ink drying
too fast or too slow. In either case, the ink
builds up on the plate. With both water-
borne and solvent-based inks, there is an
optimum temperature at which the ink will
print best. One of the major causes of ink
drying on the plate is the presence of stray
air from the in-between dryers. This air
blowing on the plates causes the ink to dry
and eventually build up until it prints in the
nonimage area. Checking the in-between

dryers to ensure they are balanced will
address this concern. The key to avoiding
ink resolubility problems is to provide prop-
er drying conditions.
Climatic Effects on
Retained Solvents
Poor drying of either water- or solvent-
based inks will result in higher levels of
retained solvents. This becomes a bigger
problem as the number of ink traps increas-
es. These increased traps cause the first-
down inks to hold onto their solvent in the
in-between dryers and hamper the removal
of the trapped solvents in the final tunnel
dryer. This can be minimized with proper
solvent selection. The key is to have the
slowest solvent in the solvent blend be a true
solvent for the ink.
It is not unusual to have retained solvent
levels higher than desirable, due to skinning
on the ink surface because the web tempera-
ture is too high. Reducing web temperature
is the first step to correct this problem. If not
successful, the solvent blend in the inks
should be adjusted. This would include a
review of true solvents for the resin system.
Water-based inks printed in high humidity
conditions tend to also show higher retained-
solvent levels, due to poor drying. One suc-
cessful approach is to treat the air going into
the ovens by removing some of the moisture.
Climatic Effects on Press Speeds
Water-borne inks generally will print at
optimum press speeds with higher tempera-
tures and lower humidity. At a certain point,
excessive temperatures can cause either
skinning over on the web, or ink drying and
building up on the printing plates. Optimum
temperature and humidity should be cou-
pled with maximum air circulation to
achieve optimum drying results. Solvent-
based inks can also experience ink buildup
on the printing plates if the air temperature
is too high. The window of acceptable tem-
perature and humidity, however, is greater
with solvent-based inks than with water-
based inks.
UV FLEXO INKS
Ultraviolet flexographic inks differ signifi-
cantly in composition from solvent-based
inks (Table 14). Most inks contain pigments
and special additives, but what separates UV
inks from conventional solvent- or water-
based inks is the use of oligomers,
monomers and photoinitiators. The oligo-
mer is the resin, or vehicle, of the UV ink.
The functional properties are dictated by the
choice of oligomers and monomers.
Unlike conventional inks, the press proper-
ties of UV inks are not sacrificed by attempt-
ing to achieve superior end use or perfor-
mance characteristics. This is due to the ink’s
capacity for intermolecular bonding or
crosslinking. Another unique trait of UV inks
is that their superior functional properties
can be modified by the addition of mono-
mers. Monomers, like solvents in convention-
al inks, are used to adjust the viscosity of an
ink. Unlike their solvent counterparts,
monomers do not evaporate, but rather cross-
link and become part of the cured ink film.
Photoinitiators are molecules that absorb
UV energy and then use that energy to initi-
ate a polymer chain reaction. The two most
common photochemical mechanisms used in
UV flexo inks are free radical and cationic.
They each have their advantages and disad-
vantages, so it is important to look into both
chemistries when choosing a UV ink.
UV Curing
After the ink is printed on the substrate, it
is passed under a source of UV energy, typi-
cally a UV-curing system. The UV-curing sys-
tem is composed of a UV lamp, a reflector, a
power supply and a control unit. The lamp is
a transparent quartz tube filled with an inert
gas, typically argon and a small amount of
INK 99
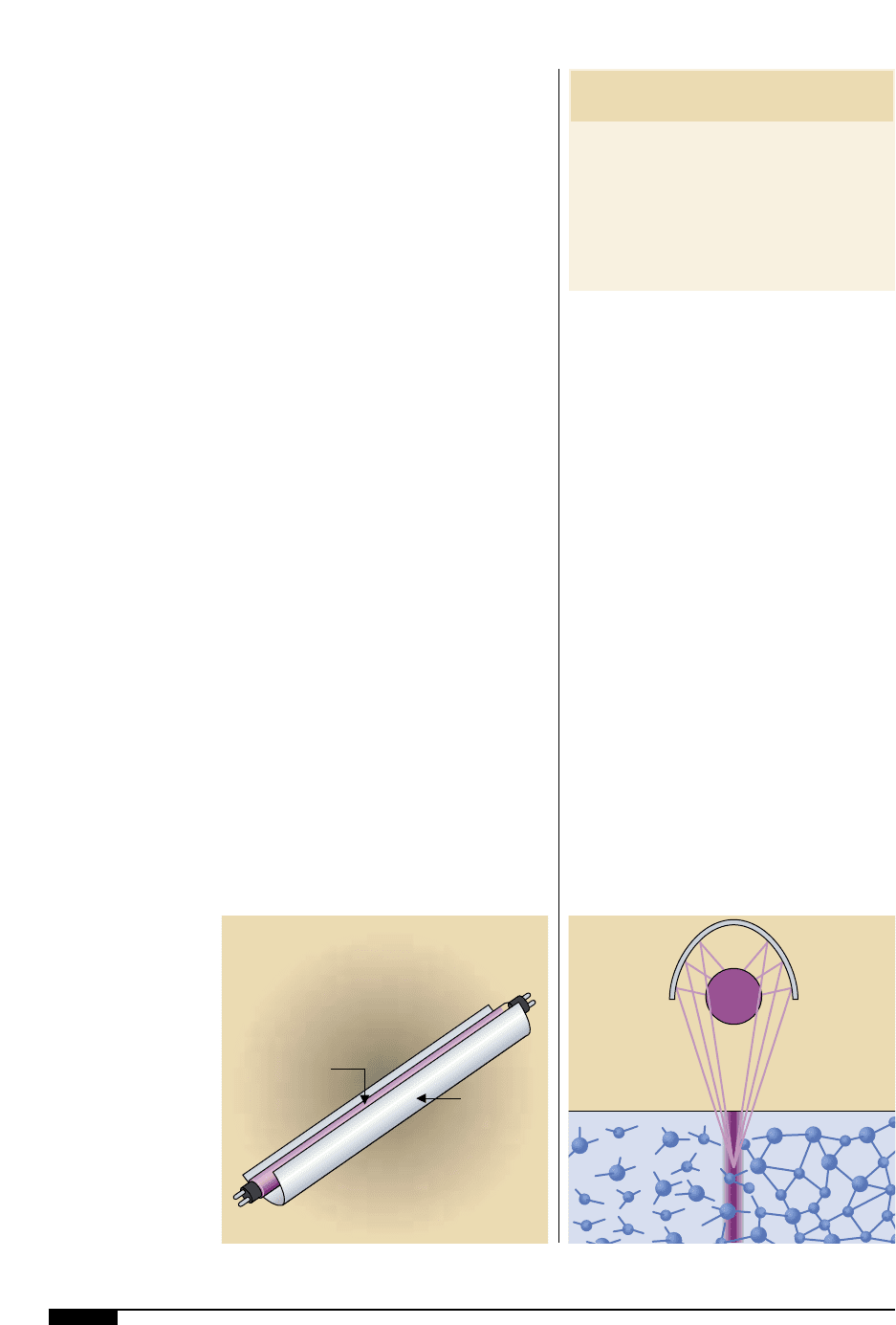
100 FLEXOGRAPHY: PRINCIPLES & PRACTICES
mercury (Figure
7&
). Quartz is used because
it is transparent to UV, whereas normal glass
is not. Mercury is used because of its strong
emissions in the ultraviolet range. The sys-
tems are rated in watts per linear inch.
Newer systems are normally 400 to 600
watts per linear inch. The reflectors for UV-
curing units typically employ the elliptical or
focused geometry to optimize the energy
delivered to the chemistry (Figure
7*
). This
focused UV energy is then absorbed by the
photoinitiators. The photoinitiators trans-
form into free radicals for the acrylate chem-
istry or Bronsted acids for the cationic
chemistry (Table 15).
Free-radical and cationic inks utilize a
chemically different set of oligomers and
monomers, so the end properties are also
affected. Free-radical acrylate chemistry is
based on acrylate-modified epoxies, ure-
thanes, polyesters and other materials. Co-
initiators are added to prevent oxygen inhi-
bition of the free-radical curing. Cationic
chemistry is based on epoxies, which cross-
link when reacting to acids. The initiator in
cationic curing is a blocked-acid catalyst
which is released by UV energy. There are
advantages and disadvantages to each chem-
istry (Table 16).
Despite many concerns about embracing
what they consider as experimental new
technology, printers should be aware that UV
is not new and not experimental. Ultraviolet
inks have been commercially available for
more than 25 years. The flexo market – espe-
cially the domestic wide-web flexo market –
is just the next printing method to find a use
for the technology. UV is the next step in the
natural progression of flexographic printing
technology, just as it was in offset lithogra-
phy, and perhaps just as it will be in gravure.
In the simplest terms, UV-curing is nothing
more than a different way to dry inks on a
printing press.
UV- vs. Solvent-based Inks
Solvent-based inks certainly have their
advantages. They are typically easy to clean
up, and wet out most printing substrates
well. The biggest advantage of solvent flexo
technology, however, is the comfort level it
7&
A standard UV lamp is a
transparent quartz tube
filled with an inert gas,
typically argon and
a small amount of
mercury.
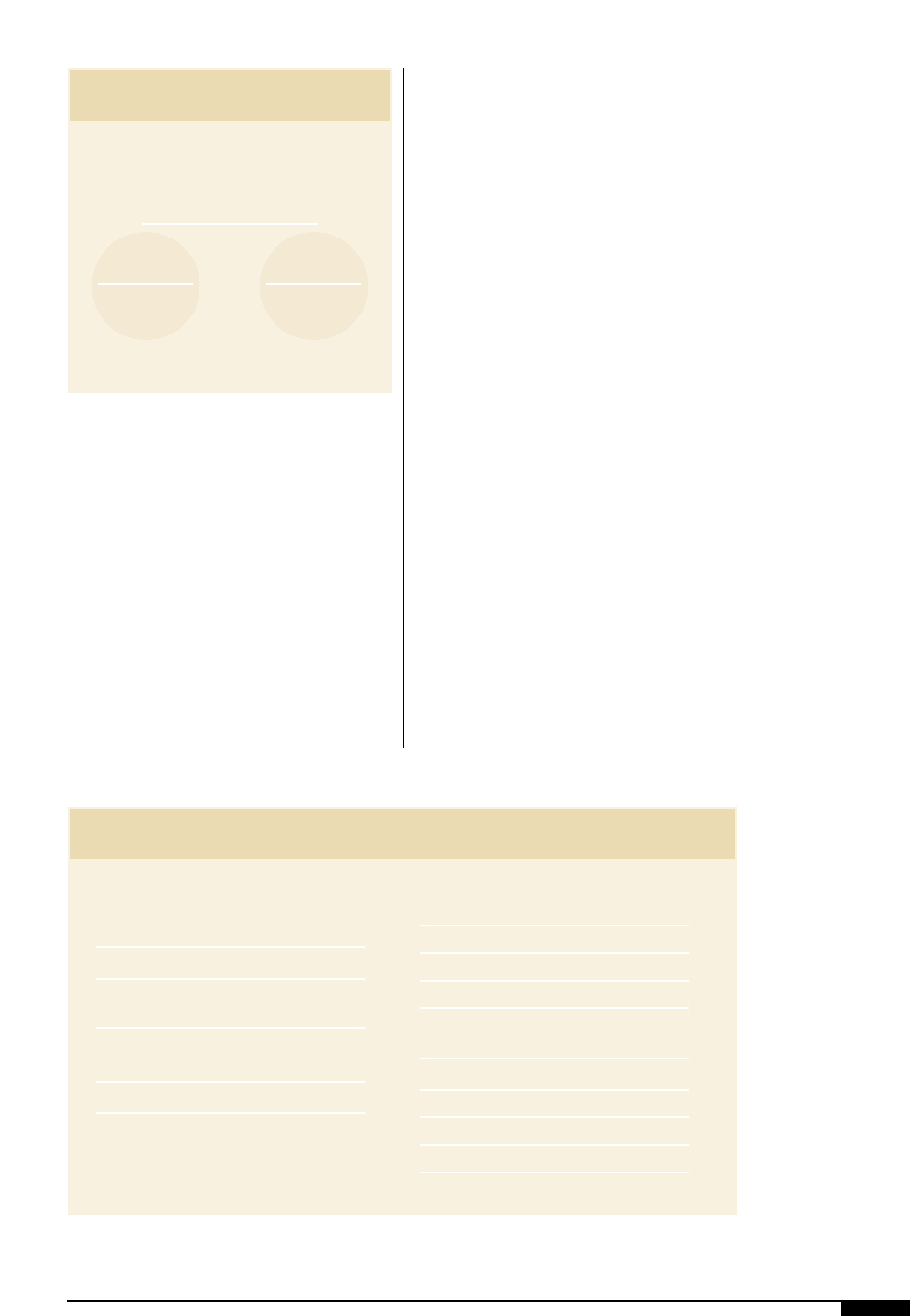
7*
Reflectors for UV-curing
units optimize the
energy delivered to the
chemistry. This focused
UV energy is then
absorbed by the pho-
toinitiators. The pho-
toinitiators evolve into
free radicals for the
acrylate chemistry or
Bronsted acids for the
cationic chemistry.
Polished
Metal
Reflector
Mercury Vapor Lamp
(Quartz Tube)
7& 7*
Table 14
CONVENTIONAL INK UV INK
Pigments Pigments
Solvents Monomers
Resins Oligomers
Additives Additives
Photoinitiators
INK COMPOSITION
inspires for both press operators and press-
room managers. Ink suppliers have been
fine-tuning these formulations for 40 years.
This is the tried-and-true technology. It has
worked in the past, so it will surely work in
the future. On the downside are VOCs. You
can burn them, you can collect them, but
you can’t ignore the environmental reper-
cussions of solvents. Using this technology
is only going to be more and more difficult
as we proceed into the 21st century.
On porous substrates like paper, water-
based inks are hard to beat. Overall equip-
ment costs can be lower since explosion-
proof wiring, solvent incineration or, for that
matter, UV lamps are not required. And
water inks are at least perceived by the lay-
man to be the most benign. On nonporous
substrates, ink formulators continue to
explore better ways to wetout the stock.
Compared to the solvent technology, water-
based ink technology is relatively new.
Unless the laws of nature can be fooled and
water evaporates as if it is solvent, then
water-based inks may be limited for this
application.
What are the advantages of UV inks? The
most obvious are the environmental bene-
fits. By eliminating solvents, printers can
enjoy two bonuses: removal of a potential
fire hazard and EPA compliance. Local,
state, and federal EPAs have wholeheartedly
embraced the UV-curing concept. Several
printers have commented that the EPA
seemed positively overjoyed at the prospect
that the printers were considering a conver-
sion from solvent to UV. Since UV inks don’t
dry until they are passed under a UV lamp,
the ink stays completely open. Unsightly
INK 101
Table 15
UV POLYMERIZATION MECHANISMS
ACRYLATE & CATIONIC
UV POLYMERIZATION MECHANISMS
Ultraviolet Energy Photoinitiation
Free Radicals Bronsted Acids
Acrylate Chemistry Cationic Chemistry
Monomer/Oligomer Monomer/Oligomer
Cured Polymer Cured Polymer
Table18
DISADVANTAGES
■ Cationics are relatively new to flexo and
require differenent handling
■ Amines will poison the cure of cationics
■ Amine functional substrates are prob-
lematic
■ Amine funcitonal pigments cannot be
used (fluorescents)
■ Perceived higher pricing
■ For CI press, speeds of white ink limited
by cure time after UV lamps
ADVANTAGES
■ Low potiential skin irritaion
■ Not oxygen inhibited
■ True metallic pigments can be used
■ Static dissipation
■ Reduced shrinkage – improved adhesion
to films
■ Post cure
■ Low odor
■ Food package: LD
50
’s are known
■ Product shelf live
■ Inherent low viscosity
CATIONIC WITH ACRYLATE CHEMISTRY
