Franc J-P. Fundamentals of Cavitation
Подождите немного. Документ загружается.

1 - INTRODUCTION – THE MAIN FEATURES OF CAVITATING FLOWS 11
where cavitation inception is expected. If T is the operating temperature of the liquid
and Dp a pressure difference that characterizes the system, the cavitation number
(also called cavitation parameter, or T
HOMA cavitation number) is defined by:
s
v
rv
ppT
p
=
- ()
D
(1.2)
For example:
— in the case of a gate:
s
v
downstream v
upstream downstream
ppT
pp
=
-
-
()
(1.3)
— for a foil placed at a submersion depth h in a horizontal free surface channel
where the pressure on the surface is p
0
and the flow velocity U:
s
r
r
v
v
pghpT
U
=
+-
0
2
1
2
()
(1.4)
— for a pump:
s
r
v
inlet v
p
ppT
V
=
- ()
2
(1.5)
where V
p
is the velocity at the periphery of the runner.
It should be noted that the cavitation number is defined using dynamical parameters
and not geometrical ones.
In a non-cavitating flow, this non-dimensional parameter cannot be considered as a
scaling parameter. The difference
pp
rv
-
has no physical significance for a single
phase flow as it cannot be obtained by integration of the pressure gradient along
a real path. The cavitation parameter becomes a similarity parameter only at
cavitation inception.
1.4.2. CAVITATION NUMBER AT INCEPTION,
ss
ss
VI
The number s
vi
is the value of the parameter s
v
corresponding to cavitation
inception at any point of the flow system. Cavitation appears because of either
a decrease in pressure at the reference point (i.e., the ambient pressure) or an
increase in the Dp-value. For experimental convenience (in particular for improved
repeatability), the number s
vd,
corresponding to cavitation disappearance from an
initial regime of developed cavitation, may also be used.
Operation in non-cavitating conditions requires that :
ss
vvi
>
(1.6)

FUNDAMENTALS OF CAVITATION12
The threshold s
vi
depends on all the usual factors considered in fluid mechanics
such as flow geometry, viscosity, gravity, surface tension, turbulence levels, thermal
parameters, wall roughness and the gas content of the liquid in terms of dissolved
and free gases (i.e., gas nuclei, see chap. 2).
In general, the smaller the value of s
vi
for a given system, the better behaved is the
flow. For example, for the flow around a 10 mm diameter circular cylinder, the
cavitation inception number is about 1.5, whereas for elliptical cylinders at zero
incidence, with a chord length of 80 mm and axis ratios of 1/4 and 1/8, the
s
vi
-values are 0.45 and 0.20, respectively.
When s
v
becomes smaller than s
vi
, cavitation usually becomes increasingly
developed. Very exceptionally, it may happen that after initial development,
cavitation finally disappears as the consequence of a further lowering of s
v
(see
chap. 6 and 8).
In many circumstances, particularly for the numerical modeling of cavitating flows,
the following estimate is taken for s
vi
:
s
vi
Cp=-
min
(1.7)
where Cp
min
is the minimum pressure coefficient, which is normally negative. The
pressure coefficient Cp at a point M is defined by the relation:
Cp
pp
p
Mr
=
-
D
(1.8)
In this expression, p
r
is the absolute pressure at the reference point, as in
relation (1.2). Two assumptions lie behind equation (1.7). First, cavitation occurs
at the point of minimum pressure and second, the pressure threshold value is that
of the vapor pressure. Clearly, these assumptions may be over-restrictive. Thus,
the estimate (1.7) must be considered cautiously.
1.4.3. RELATIVE UNDERPRESSURE OF A CAVITY,
ss
ss
C
If a developed cavity is attached to the low-pressure surface of a blade, or if a large
number of bubbles are present, the pressure in the region covered by the cavity is
uniform. Referring to this pressure as p
c
, a non-dimensional parameter known as
the relative underpressure of the cavity is defined as:
s
c
rc
pp
p
=
-
D
(1.9)
It is a true scaling parameter, as the numerator expresses an actual pressure
difference inside the flow domain. This number is extensively used in the numerical
modeling of flows with developed cavities and plays an important role in cavity
dynamics. It is often, but improperly, referred to as the cavitation number.

1 - INTRODUCTION – THE MAIN FEATURES OF CAVITATING FLOWS 13
Usually, the pressure p
c
in the cavity is the sum of two components: the vapor
pressure p
v
and a partial pressure p
g
due to the presence of non-condensable gas
inside the cavity. If this last term is negligible, the relative underpressure of the
cavity s
c
becomes equal to the cavitation parameter s
v
, which probably explains
the confusion referred to above.
1.5. SOME HISTORICAL ASPECTS
The word "cavitation" appeared in English scientific literature at the end of the
nineteenth century. It seems that the problem of cavitation in rotating machinery
handling liquids was identified by T
ORRICELLI, and later by EULER and NEWTON. In
the middle of the nineteenth century, D
ONNY and BERTHELOT measured the
cohesion of liquids. The negative effect of cavitation on the performance of a ship
propeller was first noted by P
ARSONS (1893), who built the first cavitation tunnel.
The cavitation number was introduced by T
HOMA and LEROUX around the years
1923-1925.
Subsequently, many experiments were carried out to study the physical aspects of
the phenomenon and to examine its effects on industrial systems. Theoretical and
numerical approaches were widely used. There were two main fields of research.
The first focused on bubble dynamics [R
AYLEIGH 1917, LAMB 1923, COLE 1948,
B
LAKE 1949, PLESSET 1949]. The simplicity of the spherical shape made their studies
(either theoretical or experimental) relatively easy. A large amount of work has
been published on bubble dynamics.
The second field was related to developed cavities or supercavities and was based
on the old wake theory [H
ELMHOLTZ 1868, KIRCHHOFF 1869, LEVI-CIVITA 1907,
V
ILLAT 1913, RIABOUCHINSKI 1920
3
]. This theory considers wakes as regions of
uniform pressure, limited by surfaces on which the tangential velocity is not
continuous. It is more suited to cavitating wakes than to single phase wakes. Later,
T
ULIN (1953) and WU (1956) made use of linearization procedures to adapt the
theory to the case of slender bodies such as wings and blades.
Vortical cavitation was only considered more recently, in particular by G
ENOUX
and CHAHINE (1983) and by LIGNEUL (1989), who studied the cavitating torus and
tip vortices, respectively.
3. Those references can be found in JACOB's book: Introduction mathématique à la mécanique
des fluides.
FUNDAMENTALS OF CAVITATION14
REFERENCES
BIRKHOFF G. & ZARANTONELLO E.H. –1957– Jets, wakes and cavities.
Academic Press Inc.
B
LAKE F.G. -1949- The tensile strength of liquids: a review of the literature.
Harvard Acoustics Res. Lab. TM 9, June.
C
OLE R.H. -1948- Underwater explosions. Princeton University Press.
G
ENOUX P. & CHAHINE G.L. -1983- Équilibre statique et dynamique d'un tore de
vapeur tourbillonnaire. J. Méc. Théor. Appl. 2(5), 829-857.
J
ACOB C. -1959- Introduction mathématique à la mécanique des fluides.
Gauthier-Villars Ed.
KNAPP R.T., DAILY J.W. & HAMMITT F.G. –1970– Cavitation. McGraw-Hill Book
Company Ed., 578 p.
L
AMB H. -1923- The early stages of a submarine explosion. Phil. Mag. 45, 257 sq.
L
ESIEUR M. -1998- Vorticity and pressure distributions in numerical simulations
of turbulent shear flows. Proc. 3
rd
Int. Symp. on Cavitation, vol. 1,
Grenoble (France), April 7-9, 9-18.
L
EVKOVSKY Y.L. –1978– Structure of cavitating flows. Sudostroenie Publishing House,
Leningrad (Russia), 222 p.
L
IGNEUL P. -1989- Theoretical tip vortex cavitation inception threshold.
Eur. J. Mech. B/Fluids 8, 495-521.
P
ERNIK A.D. –1966– Problems of cavitation. Sudostroenie Publishing House,
Leningrad (Russia), 439 p.
P
LESSET M.S. -1949- The dynamics of cavitation bubbles. J. Appl. Mech. 16, 277 sq.
R
AYLEIGH (Lord) -1917- The pressure developed in a liquid during the collapse of
a spherical cavity. Phil. Mag. 34, 94 sq.
R
OZHDESTVENSKY V.V. –1977– Cavitation. Sudostroenie Publishing House,
Leningrad (Russia), 247 p.
TULIN M.P. -1953- Steady two-dimensional cavity flows about slender bodies.
DTMB, Rpt 834.
W
U T.Y.T. -1956- A free streamline theory for two-dimensional fully cavitated
hydrofoils. J. Math. Phys. 35, 236-265.
2. NUCLEI AND CAVITATION
2.1. INTRODUCTION
2.1.1. LIQUID TENSION
In chapter 1, possible differences between the actual value of the cavitation
threshold and the vapor pressure were discussed. In real flows as in laboratory
flows, liquids can actually sustain absolute pressures lower than the vapor
pressure at the operating temperature and even negative pressures, i.e. tensions.
To explain these discrepancies, one must first refer to the classical data relating to
liquid breakdown. In the nineteenth century [D
ONNY 1846, BERTHELOT 1850,
R
EYNOLDS 1882], experiments demonstrated that a liquid at rest could sustain
negative pressures without vaporization occurring. For water, the values were of
the order of several tens of bars. More recent experiments [T
EMPERLEY 1946, BRIGGS
1950, REES & TREVENA 1966] have shown that the experimental values are rather
scattered (for example, B
RIGGS obtained 277 bars). They depend on the experimental
procedure, the preliminary treatment of the liquid (for example, degassing or
pressurization over a long period) and the degree of cleanliness of the container
wall. It is often not clear whether the limit corresponds to a loss of cohesion in the
bulk liquid or a loss of adhesion of the liquid to the walls.
These experimental values are lower than the estimates calculated from theoretical
models. For example, considering a microscopic bubble with a diameter of the order
of the intermolecular distance d
0
, the expression
2
0
Sd/
(S is the liquid surface
tension) gives a value of 7,000 bars (with
d
0
01ª .nm
, S = 0.072 N/m for water).
This estimate is obviously open to criticism, as such a very small size is not
compatible with the assumption of a continuum. If the molecular nature of the
liquid is then taken into account, together with oscillations due to temperature
fluctuations, the limiting tension is reduced by about one order of magnitude.
Another estimate can be derived from the V
AN DER WAALS equation (see § 1.1.2,
fig. 1.2). The ordinate of the minimum M on the isotherm can be considered a
limiting value of the tension sustained by a liquid. In the case of water, it is about
500 bars at room temperature.
2.1.2. CAVITATION NUCLEI
The differences observed with respect to the vapor pressure p
v
(T) in typical
experiments on cavitation are much smaller than the experimental results and
FUNDAMENTALS OF CAVITATION16
theoretical estimates mentioned above. They don’t usually exceed a few bars at
most for tap water. Thus, for liquids currently used in industry, the existence of
points of weakness in the liquid continuum is to be expected. Those points are
formed by small gas and vapor inclusions and operate as starting points for the
liquid breakdown. They are known as cavitation nuclei. Numerous experiments
show that those nuclei actually exist. Their size is between a few micrometers and
some hundreds of micrometers. They remain spherical at this scale due to surface
tension. They can be referred to as microbubbles.
The assumption of heterogeneities inside a homogeneous medium in order to
explain phase changes is common in thermophysics, for example in boiling,
condensation, and solidification. Nuclei also proved to be the origin of great
differences in cavitation inception found in the past when tests on similar bodies
were made in different facilities.
Various questions arise concerning nuclei: How do they appear? Are they stable?
What is their effect on liquid cohesion and then on conditions of cavitation
inception? How can they be measured? How can the nucleus content of a liquid be
characterized?
Nuclei are present either on walls or in the liquid bulk. Surface nuclei consist of gas
trapped in small wall crevices that are not filled with the liquid (see § 2.3.2). The
wetting capacity of the liquid is therefore of great importance. It is possible that
bulk nuclei are produced by cosmic rays, i.e., by a mechanism of energy deposition
similar to the one used in bubble chambers for the experimental study of atomic
particles. Another example of micronic bubble production by energy deposition is
found in the breakdown of insulating liquids subjected to high voltage [A
ITKEN et al.
1996, J
OMNI et al. 1999]. However, the most efficient way to produce nuclei is the
reduction in pressure of a saturated liquid. Regions downstream of developed
cavities may also be an abundant source of nuclei, as it will be seen in chapter 7.
Once present, nuclei evolve under two main influences. First, free nuclei (i.e., not
attached to a wall) rise due to gravity. Second, all nuclei exchange gas via diffusion
with the dissolved gas present in the surrounding liquid. In general, as the mass
diffusion coefficients are very small, the diffusion process is slow and typical
diffusion times are long, of the order of a second (see § 2.3.2). This is a large value
in comparison with the time necessary for bubble collapse, which typically takes
milliseconds (see chap. 3). Thus, in the following section, mechanical equilibrium
of a spherical nucleus is assumed and the mass of enclosed gas is supposed
constant. The problem of gas diffusion will be considered at the end of the chapter
as it concerns the stability of gas nuclei over a long period.
The void fraction resulting from the presence of free nuclei is extremely small. For
example, for a concentration of one hundred nuclei per cubic centimeter (this value
is actually rather high) with a diameter of 0.1 mm, the void fraction is 0.52
¥ 10
–4
.
Thus, the liquid density remains practically unchanged. The same conclusion holds
for the velocity of sound in the liquid.
2 - NUCLEI AND CAVITATION 17
2.2. EQUILIBRIUM OF A NUCLEUS
2.2.1. EQUILIBRIUM CONDITION [BLAKE 1949]
Consider a spherical microbubble, containing gas and vapor, in equilibrium within
a liquid at rest. The liquid is supposed capable of withstanding pressures below
the vapor pressure p
v
and even tensions. Its metastable state can be represented by
a point on the branch AM of the V
AN DER WAALS curve in figure 1.2. The microbubble
is the point of weakness from which the breakdown of the liquid medium can begin.
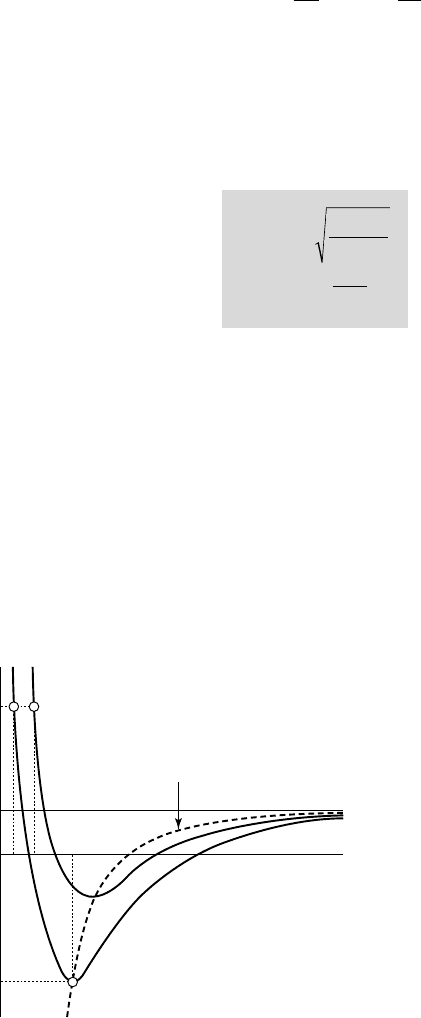
2.1
Microbubble in a liquid
The bubble radius R is considered sufficiently
small for the difference in hydrostatic pressure
2r gR to be negligible compared to the pressure
difference corresponding to the surface tension
2S R/
. This condition requires that R be smaller
than the limiting value
(S/ g)r
, namely 2.7 mm
for water. It is fulfilled in this case since micro-
microbubbles of diameter smaller than about 0.5 mm only are considered. The
pressure can then be considered uniform in the bulk of the surrounding liquid
(expressed as p
•
) and the microbubble is actually spherical.
The equilibrium of the interface requires the following condition to be satisfied:
ppp
S
R
gv•
=+-
2
(2.1)
in which p
g
is the partial pressure of the gas inside the bubble, S the surface tension
and R the radius.
It is assumed that the pressure changes slowly so that mechanical equilibrium is
still satisfied and that heat transfer between gas and liquid is possible. However,
the change in pressure must be rapid enough to ensure that gas diffusion at the
interface is negligible. In other words, the transformation is assumed isothermal
and the mass of gas constant.
For the initial state, denoted by subscript 0, equation (2.1) is written:
ppp
S
R
gv•
=+-
00
0
2
(2.2)
R
p
v
+p
g
p
∞
FUNDAMENTALS OF CAVITATION18
As the gas pressure is inversely proportional to the volume in an isothermal
transformation, then from equation (2.1) one obtains:
pp
R
R
p
S
R
gv•
=
È
Î
Í
˘
˚
˙
+-
0
0
3
2
(2.3)
The curve p
•
(R) is shown in figure 2.2. The two mechanisms considered in
equation (2.1), i.e.,
— the internal pressure effect, which tends to increase the bubble size, and
— the surface tension effect, which acts in the opposite direction, result in the
existence of a minimum given by:
RR
p
SR
pp
S
R
c
g
cv
c
=
=-
Ï
Ì
Ô
Ô
Ó
Ô
Ô
0
0
0
3
2
4
3
/
(2.4)
The corresponding locus is also represented in figure 2.2.
For a given liquid and a given gas at a fixed temperature, a nucleus is characterized
by the mass of gas it contains, which is proportional to the quantity p
g0
R
0
3
. To define
a nucleus with a constant mass of gas, one can use either one of the doublets
(R
0
,p
•
), (R
0
,p
g0
), etc. or preferably one of the quantities R
c
or p
c
.
2.2.2. STABILITY AND CRITICAL PRESSURE OF A NUCLEUS
Consider another nucleus containing more gas and having a radius
RR'
00
>
under
pressure p
•0
. The equilibrium curve of this nucleus is shown in figure 2.2. The
corresponding gas pressure p'
g0
is smaller than p
g0
, as seen in equation (2.2).
2.2
Equilibrium of a spherical nucleus
Suppose a virtual transformation is
carried out resulting in the radius
of the first nucleus becoming R'
0
so
that the surface tension terms are
identical. In its new state, the first
nucleus has a gas pressure given
by
pRR
g0 0 0
3
(/')
. This is lower
than p'
g0
as can be seen from a
comparison of the mass of gas
enclosed in each nucleus. This is
R
locus of minima
R
c
R
0
R'
0
p
∞
p
∞0
p
c
p
v
0
2 - NUCLEI AND CAVITATION 19
proportional to p
g0
R
0
3
. Thus, the balance of forces tends to return the first nucleus
to its initial state. Hence, the mechanical equilibrium of the spherical nucleus is
stable on the branch of the curve that has a negative slope. It is straightforward to
check that the equilibrium is unstable on the other branch.
The minimum pressure, p
c
, that the nucleus can withstand under stable conditions
is a limiting value referred to as the critical pressure. The difference
pp
vc
-
is the
static delay to cavitation. Expression (2.4) suggests that the smaller the nucleus, the
greater the delay.
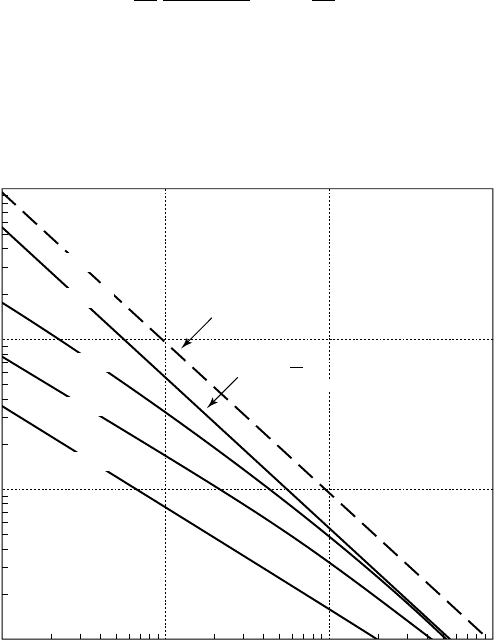
Figure 2.3 gives another representation of the equilibrium curves, limited to the
stable domain, in the case of air nuclei in water. It is based on the following
equation:
p
SR
pp
p
S
R
vc
v•
=
-
+-
4
27
2
2
3
2
(/)
()
obtained by combination of equations (2.3) and (2.4). The evolution of a given
nucleus, characterized by its critical pressure, under varying external pressures, can
be followed on a vertical line. On this diagram, the larger nuclei, which are more
likely to provoke a breakdown of the liquid medium, have the smaller static delay.
1000
100
10
1
0.001 0.01 0.1 1
Static delay p
v
– p
c
[bars]
Nuclei radius [micrometers]
p
∞
= p
c
p
∞
= p
v
p
∞
= 0.1 bar
p
∞
= 1 bar
p
∞
= 10 bars
R
c
= – ———
S
p
v
– p
c
4
3
4
3√3
S
p
v
– p
c
R = —— ———
2.3 - Radius of equilibrium of air nuclei in water
for various external pressures p
••
••
FUNDAMENTALS OF CAVITATION20
2.2.3. NUCLEUS EVOLUTION IN A LOW PRESSURE REGION
Via BLAKE's model the evolution of an isolated nucleus passing through a low-
pressure region of limited extent is more easily understood. Consider, for example,
the evolution of a nucleus in a Venturi (fig. 2.4). The local pressure at a distance s
in this approximately one-dimensional flow is denoted p(s). For the nucleus, this
local pressure p(s) plays the role of p
•,
as introduced in section 2.2.1.
Two cases must be considered according to the value of the minimum pressure p
min
with regard to the critical pressure p
c
of the nucleus.
® If
pp
cmin
>
, the nucleus grows slightly and then returns to its initial state as it
passes through the throat.
® If
pp
cmin
<
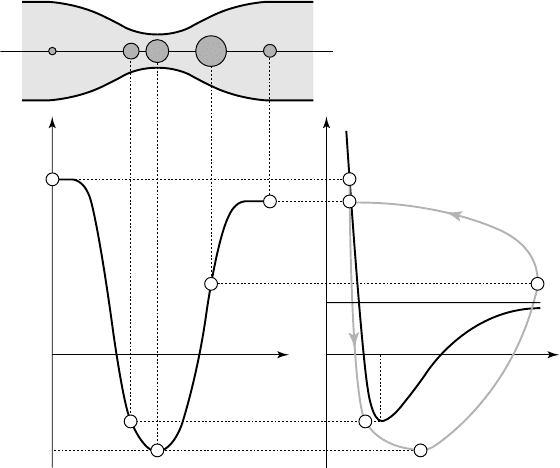
, it becomes unstable and much larger than it was initially. Figure 2.4
shows its evolution in the (R,p
•
) diagram, as deduced from dynamic calculations
(see chap. 3). Due to inertial forces, its maximum size is reached at point D, a
little downstream from throat C. At D, it is far from equilibrium, so that the
increasing external pressure compels it to collapse violently.
Throat
Throat
Throat
AB DE
pp
∞
p
v
p
c
AA
p
min
0
BB
DD
E
E
Rs
2.4 - Typical nucleus evolution in a Venturi. Unstable case: p
min
<p
c
This type of bubble evolution can be applied to many practical situations in which
the time spent in the low-pressure region is long compared to other characteristic
times, particularly the collapse time (see chap. 3 for an estimation of this time).
Note that, on the stable branch of the equilibrium curve (fig. 2.2), small oscillations
