Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c032” — 2007/4/9 — 15:53 — page 12 — #12
32-12 Tissue Engineering
[15] Kollar, E.J. and Baird, G.R. The influence of the dental papilla on the development of tooth shape
in embryonic mouse tooth germs. J. Embryol. Exp. Morphol. 21: 131–148, 1969.
[16] Kollar, E.J. and Baird, G.R. Tissue interactions in embryonic mouse tooth germs. I. Reorganization
of the dental epithelium during tooth-germ reconstruction. J. Embryol. Exp. Morphol. 24: 159–171,
1970.
[17] Maas, R. and Bei, M. The genetic control of early tooth development. Crit. Rev. Oral Biol. Med. 8:
4–39, 1997.
[18] Vainio, S. et al. Identification of BMP-4 as a signal mediating secondary induction between epithelial
and mesenchymal tissues during early tooth development. Cell 75: 45–58, 1993.
[19] Chen, Y. et al. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development
122: 3035–3044, 1996.
[20] Kratochwil, K. et al. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions
in tooth and hair development. Genes Dev. 10: 1382–1394, 1996.
[21] Neubüser, A. et al. Antagonistic interactions between FGF and BMP signaling pathways: a
mechanism for positioning the sites of tooth formation. Cell 90: 247–255, 1997.
[22] Bitgood, M.J. and McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites
of cell-cell interaction in the mouse embryo. Dev. Biol. 172: 126–138, 1995.
[23] Koyama, E. et al. Polarizing activity, Sonic hedgehog, and tooth development in embryonic and
postnatal mouse. Dev. Dyn. 206: 59–72, 1996.
[24] Hardcastle, Z. et al. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3
mutants. Development 125: 2803–2811, 1998.
[25] Vaahtokari, A. et al. The enamel knot as a signaling center in the developing mouse tooth. Mech.
Dev. 54: 39–43, 1996a.
[26] Thesleff, I. and Jernvall, J. The enamel knot: a putative signaling center regulating tooth
development. Cold spring harbour Symp. Quant. Biol. 62: 257–267, 1997.
[27] Vaahtokari, A., Åberg, T., and Thesleff, I. Apoptosis in the developing tooth: association with an
embryonic signaling center and suppression by EGF and FGF-4. Development 122: 121–129, 1996b.
[28] Kettunen, P. and Thesleff, I. Expression and function of FGFs-4, -8, and -9 suggest functional
redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev. Dyn. 211:
256–268, 1998.
[29] MacKenzie, A., Ferguson, M.W., and Sharpe, P.T. Expression patterns of the homeobox gene,
Hox-8, in the mouse embryo suggest a role in specifying tooth initiation and shape. Development
115: 403–420, 1992.
[30] Iseki, S. et al. Sonic hedgehog is expressed in epithelial cells during development of whisker, hair,
and tooth. Biochem. Biophys. Res. Commun. 218: 688–693, 1996.
[31] Satokata, I. and Maas, R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial
and tooth development. Nat. Genet. 6: 348–356, 1994.
[32] van Genderen, C. et al. Development of several organs that require inductive epithelial-
mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8: 2691–2703, 1994.
[33] Peters, H., Neubüser, A., and Balling, R. Pax genes and organogenesis: Pax9 meets tooth
development. Eur. J. Oral Sci. 106: 38–43, 1998.
[34] Matzuk, M.M., Kumar, T.R., and Bradley, A. Different phenotypes for mice deficient in either
activins or activin receptor type II. Nature 374: 356–360, 1995.
[35] Tucker, A.S., Al Khamis, A., and Sharpe, P.T. Interactions between Bmp-4 and Msx-1 act to restrict
gene expression to odontogenic mesenchyme. Dev. Dyn. 212, 533–539, 1998.
[36] Vieira, A.R. Oral clefts and syndromic forms of tooth agenesis as models for genetics of isolated
tooth agenesis. J. Dent. Res. 82: 162–165, 2003.
[37] Lammi, L. et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal
cancer. Am. J. Hum. Genet. 74: 1043–1050, 2004. Epub 2004 Mar 23.
[38] Thomas, E.D. Bone marrow transplantation from bench to bedside. Ann. NY Acad. Sci. 770: 34–41,
1995.
mikos: “9026_c032” — 2007/4/9 — 15:53 — page 13 — #13
The Bioengineering of Dental Tissues 32-13
[39] Snowden, J.A. et al. Autologous hemopoietic stem cell transplantation in severe RA: a report from
the EBMT and ABMTR. J. Rheumatol. 31: 482–488, 2004.
[40] Rini, B.I. et al. Allogeneic stem-cell transplantation of renal cell cancer after nonmyeloablative
chemotherapy: feasibility, engraftment, and clinical results. J. Clin. Oncol. 20: 2017–2024, 2002.
[41] Gronthos, S. et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl
Acad. Sci. USA 97: 13625–30136, 2000.
[42] Gronthos, S. et al. Stem cell properties of human dental pulp stem cells. J. Dental. Res. 81: 531–535,
2002.
[43] Shi, S., Robey, P.G., and Gronthos, S. Comparison of gene expression profiles for human, dental
pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29: 532–539,
2001.
[44] Ekholm, S.V. and Reed, S.I. Regulation of G(1) cyclin-dependent kinases in the mammalian cell
cycle. Curr. Opin. Cell Biol. 12: 676–684, 2000.
[45] Grossel, M.J., Baker, G.L., and Hinds, P.W. cdk6 can shorten G(1) phase dependent upon the
N-terminal INK4 interaction domain. J. Biol. Chem. 274: 29960–29967, 1999.
[46] Shi, S. and Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells identified in human
bone marrow and dental pulp. J. Bone Miner. Res. 18: 696–704, 2003.
[47] Batouli, S. et al. Comparison of stem cell-mediated osteogenesis and dentinogenesis. J. Dental. Res.
82: 975–980, 2003.
[48] Levin, L.G. Pulpal regeneration. Pract. Periodont. Aesthet. Dental. 10: 621–624, 1998.
[49] About, I. et al. Pulpal inflammatory responses following non-carious class V restorations. Oper.
Dental. 26: 336–342, 2001.
[50] About, I. et al. The effect of cavity restoration variables on odontoblast cell numbers and dental
repair. J. Dental. 29: 109–117, 2001.
[51] Murray, P.E. et al. Restorative pulpal and repair responses. J. Am. Dental. Assoc. 132: 482–491, 2001.
[52] Murray, P.E. et al. Postoperative pulpal and repair responses. J. Am. Dental. Assoc. 131: 321–329,
2000.
[53] Rutherford, R.B. and Gu, K. Treatment of inflamed ferret dental pulps with recombinant bone
morphogenetic protein-7. Eur. J. Oral Sci. 108: 202–206, 2000.
[54] Sloan, A.J., Rutherford, R.B., and Smith, A.J. Stimulation of the rat dentine-pulp complex by bone
morphogenetic protein-7 in vitro. Arch. Oral Biol. 45: 173–177, 2000.
[55] Rutherford, R.B. et al. Bone morphogenetic protein-transduced human fibroblasts convert to
osteoblasts and form bone in vivo. Tissue Eng. 8: 441–52, 2002.
[56] Iohara, K. et al. Dentin regeneration by dental pulp stem cell therapy with recombinant human
bone morphogenetic protein 2. J. Dental. Res. 83: 590–595, 2004.
[57] Young, C.S. et al. Tissue engineering of complex tooth structures on biodegradable polymer
scaffolds. J. Dental. Res. 81: 695–700, 2002.
[58] Duailibi, M.T. et al. Bioengineered teeth from cultured rat tooth bud cells. J. Dental. Res. 83:
523–528, 2004.
[59] Ohazama, A. et al. Stem-cell-based tissue engineering of murine teeth. J. Dental. Res. 83: 518–522,
2004.
[60] Tucker, A.S., Matthews, K.L., and Sharpe, P.T. Transformation of tooth type induced by inhibition
of BMP signalling. Science 282: 1136–1138, 1998.
[61] Ferguson, C.A., Tucker, A.S., and Sharpe, P.T. Temporospatial cell interactions regulating
mandibular and maxillary arch patterning. Development 127: 403–412, 2000.
[62] Thomas, B.L. et al. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development
124: 4811–4818, 1997.
[63] Grigoriou, M. et al. Expression of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain genes,
suggests a role in mammalian head development. Development 125: 2063–2074, 1998.
[64] Tucker, A.S. et al. Fgf-8 determines rostral–caudal polarity in the first branchial arch. Development
126: 51–61, 1999.
mikos: “9026_c032” — 2007/4/9 — 15:53 — page 14 — #14
32-14 Tissue Engineering
[65] Mitsiadis, T.A. et al. Role of Islet1 in the patterning of murine dentition. Development 130:
4451–4460, 2003.
[66] Mucchielli, M.L. et al.Otlx2/RIEG expression in the odontogenic epithelium precedes tooth ini-
tiation and requires mesenchyme-derived signals for its maintenance. Dev. Biol. 189: 275–284,
1997.
[67] St. Amand, T.R. et al. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1
and Pitx2 in mouse tooth-forming anlage. Dev. Biol. 217: 323–332, 2000.
[68] Headon, D.J. et al. Gene defect in ectodermal dysplasia implicates a death domain adaper in
development. Nature 414: 913–916, 2002.
[69] Sarkar, L. et al. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian
tooth development. Proc. Natl Acad. Sci. USA 97: 4520–4524, 2000.
[70] Ferguson, C.A. et al. Activin is an essential early mesenchymal signal in tooth development that is
required for patterning of the murine dentition. Genes Dev. 12: 2636–2649, 1998.
[71] D’Souza, R.N. et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth
development in mice. Development 126: 2911–2920, 1999.
[72] Tucker, A.S. et al. Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis.
Development 127: 4691–4700, 2000.
[73] Kettunen, P. et al. Associations of FGF-3 and FGF-10 with signaling networks regulating tooth
morphogenesis. Dev. Dyn. 219: 322–332, 2000.
[74] Kettunen, P., Karavanova, I., and Thesleff, I. Responsiveness of developing dental tissues to fibroblast
growth factors: expression of splicing alternatives of FGFR1,-2,-3, and of FGFR4; and stimulation
of cell proliferation by FGF-2,-4,-8, and -9. Dev. Genet. 22: 374–385, 1998.
[75] Åberg, T., Wozney, J., and Thesleff, I. Expression patterns of bone morphogenic proteins (bmps)
in the developing mouse tooth suggest poles in morphogenesis and cell differentiation. Dev. Dyn.
210: 383–396, 1997.
[76] Sarkar, L. and Sharpe, P.T. Expression of wnt signaling pathway genes during tooth development.
Mech. Dev. 85: 197–200, 1999.
[77] Snead, M.L., Luo, W., Lau, E.C., and Slavkin, H.C. Spatial and temporal-restricted pattern for
amelogenin gene expression during mouse molar tooth organogenesis. Development 104: 77–85,
1988.
[78] Bègue-Kirn, C. et al. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin:
tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur.
J. Oral Sci. 106: 963–970, 1998.
[79] Developmental biology programme of the University of Helsinki. Gene Expression in Tooth [online],
http://biteit.helsinki.fi, 1996.
mikos: “9026_c033” — 2007/4/9 — 15:53 — page1—#1
33
Tracheal Tissue
Engineering
Brian Dunham
Paul Flint
Sunil Singhal
Catherine Le Visage
Kam Leong
Johns Hopkins School of Medicine
33.1 Introduction.............................................. 33-1
33.2 Tracheal Reconstruction: Previous Attempts .......... 33-5
33.3 Tracheal Tissue Engineering ............................ 33-8
References ....................................................... 33-13
33.1 Introduction
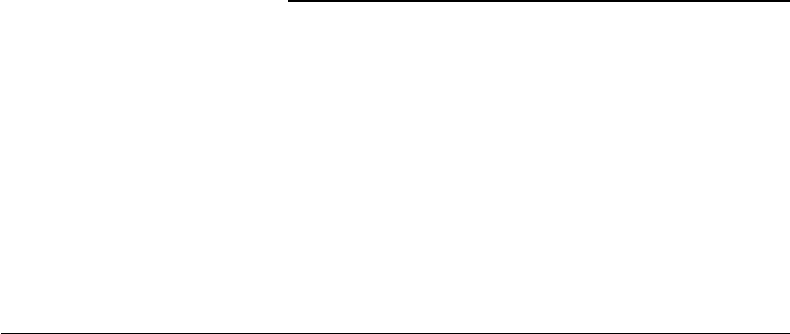
A seemingly simple, single-lumen structure, the trachea is the sole conduit between the supraglottic airway
and the lungs. Humidified and warmed air inspired through the nose travels to the lungs through the
relatively thin-walled trachea, which widens slightly at its distal end. At birth, its diameter is approximately
0.5 cm. Tracheal size grows proportionally with the height and weight of the child [1,2]. In a male
human adult, the trachea is approximately 12-cm long and 1.5- to 2-cm wide. In an adult female it is
approximately 11-cm long and narrower. At its distal end, the carina, it bifurcates into the two mainstem
bronchi (Figure 33.1).
Mechanically, the trachea has several functions. As an air conduit one of its most important structural
functions is to maintain patency; any significant obstruction of its lumen can result in rapid asphyxiation.
It must also be flexible enough to accommodate cervical rotation, flexion, and extension. Furthermore,
it has to withstand both negative and positive intraluminal pressures encountered in the respiratory cycle.
Approximately 16 to 20 hyaline cartilage rings provide the necessary rigidity; the intervening soft tissue
provides the necessary flexibility and compliance to respond to cervical motion and varying intraluminal
pressure. The first and most superior ring, the cricoid cartilage, is a complete ring (Figure 33.1). The
remaining cartilage rings beneath the cricoid are C-shaped and open posteriorly. The pars membrana
spans the open ends of the cartilage rings and is composed of a fibroelastic ligament and longitudinally
oriented smooth muscle (Figure 33.2). The ligament prevents overdistention, while contraction of the
muscle reduces the size of the lumen. The latter occurs during the cough reflex; the decreased luminal size
increases the velocity of the expired air, facilitating airway clearance.
The trachea is lined with a pseudostratified columnar respiratory epithelium that consists of a het-
erogeneous population of cells that form tight junctions; in the submucosal space are numerous mixed
33-1
mikos: “9026_c033” — 2007/4/9 — 15:53 — page2—#2
33-2 Tissue Engineering
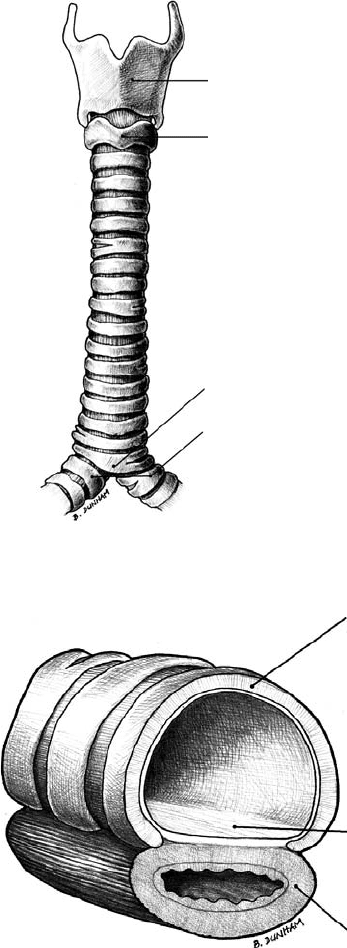
Thyroid cartilage
Cricoid cartilage
Right and left
main bronchi
Carina
FIGURE 33.1 Anterior view of a human trachea.
A
B
C
FIGURE 33.2 Cross sectional view of a human trachea segment. (A)C-shaped cartilaginous ring. (B) Pars membrana
of the trachea, which is composed of a fibroelastic ligament and smooth muscle. (C) Esophagus.
sero-mucous glands, which decrease in numbers in the distal aspect of the trachea (Figures 33.3a,b).
Airway epithelial cells, as well as dendritic cells found in airway epithelium, express major histocompatib-
ility complex class I and II molecules, which endow the epithelium with the properties of an immunologic
barrier [3]. The epithelium’s major function was once thought to be that of a physical barrier; it is
now thought to be far more complex. The airway surface epithelium does indeed possess a variety of
intercellular junctional complexes that create a tight and efficient barrier against inhaled pathogens and
other noxious agents [4,5]. In addition, airway epithelial cells act together to ensure mucosal defense
mikos: “9026_c033” — 2007/4/9 — 15:53 — page3—#3
Tracheal Tissue Engineering 33-3
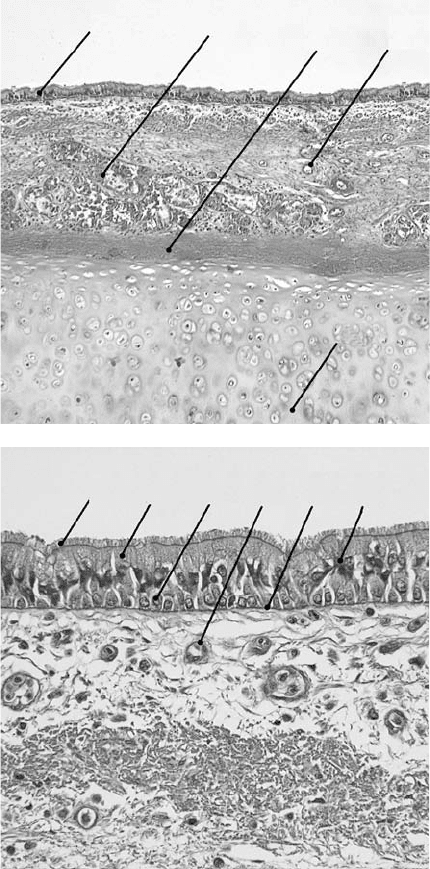
A
B
CD
E
(a)
(b)
A
B
CDEF
FIGURE 33.3 (a) Photomicrograph of tracheal tissue, hematoxylin and eosin stain ×200. (A) Respiratory epithe-
lium. (B) Mixed sero-mucous glands residing in the lamina propria. (C) Perichondrium. (D) Blood vessel. The lamina
propria underlying the epithelium is richly vascularized, which helps to warm the inspired air. (E) Cartilage. (b) Pho-
tomicrograph of tracheal respiratory epithelium, hematoxylin and eosin stain ×400. (A) Cilia arising from (B), the
columnar ciliated epithelial cells. (C) Basal cell. (D) Blood vessel within the lamina propria. (E) Basement membrane.
(F) Goblet cell.
through a variety of mechanisms such as mucociliary clearance, active secretion of ions and regulation of
water balance, regulation of airway smooth muscle function, and the release of antibacterial, antioxidant,
and anti-inflammatory molecules in the airway surface liquid. Airway epithelium constitutes the inter-
face between the internal milieu and the external environment, and responds to changes in the external
environment by secreting a large number of mediators that interact with cells of the immune system
and underlying mesenchyme [5]. These mediators include arachidonic acid metabolites, nonprostanoid
inhibitory factors, nitric oxide, endothelin, cytokines, and growth factors [3].
mikos: “9026_c033” — 2007/4/9 — 15:53 — page4—#4
33-4 Tissue Engineering
Since the epithelium is in direct and permanent contact with the external environment, it is frequently
injured. It is capable of rapid restitution if it is denuded [6,7]. After an injury, epithelial cells dedifferentiate,
flatten, and migrate rapidly beneath a fibrin–fibronectin plasma-derived gel that contains both adhesive
plasma proteins and leukocytes [8]. The response to injury, however, appears to partly depend on the depth
of injury. Deep injuries violating the lamina propria and reaching the perichondrial tissues are associated
with excessive granulation tissue [9,10]. Bacterial and viral infections, inhaled pollutants and toxic agents,
and mechanical stress can severely alter the integrity of the epithelial barrier. The response of the airway
surface epithelium to an acute injury includes a succession of cellular events varying from loss of surface
epithelial impermeability to partial shedding of the epithelium or even to complete denudation of the
basement membrane. In response to chronic injury, the airway epithelial cells can also transdifferentiate,
with a shift from serous to mucous cells, from ciliated to secretory cells, or from secretory to squamous cells.
Such a remodeling illustrates the marked plasticity and capacity of the airway epithelium to regenerate
[4,11]. Given its regenerative capacity, characterization of airway stem cells may eventually lead to clinical,
therapeutic benefit [12].
There are at least eight morphologically distinct cells types in human respiratory epithelium. These
include columnar ciliated epithelial cells, mucous goblet cells, serous cells, basal cells, Clara cells, pul-
monary endocrine cells, as well as intraepithelial nerve cells, and a variety of immune cells. The latter
group of cells is comprised of mast cells, intraepithelial lymphocytes, dendritic cells, and macrophages.
Serous cells and Clara cells are found beyond the trachea in the more distant airway conduits. The most
abundant of the tracheal epithelial cells are the ciliated columnar cells, accounting for approximately 50%
of all epithelial cells. Ciliated cells, which arise from either basal or secretory cells, are no longer thought
to be terminally differentiated [5,13]. In the adult human trachea, each of these ciliated columnar cells
host approximately 300 cilia that beat in an organized fashion to sweep respiratory secretions upward into
the larynx and oral cavity.
The second most common cell in the human trachea is the mucous goblet cell, which is characterized by
acidic-mucin granules. Secretion into the airway lumen of the correct amount of mucin, a glycoprotein,
and the viscoelasticity of the resulting mucus are important parameters for an efficient mucociliary
clearance of mucus-entrapped foreign bodies. It is thought that the acidity, due to the sialic acid content
of the glycoprotein, determines the viscoelastic profile and hence the relative ease of transport across cilia
[5]. These goblet cells are thought to be capable of self-renewal and may differentiate into ciliated cells
[14,15], as do the basal cells [16]. The basal cells are short, rounded cells that lie on the basal lamina
without extension to the apical surface. They are the only cells in the epithelium that are firmly attached
to the basement membrane and, as such, aid in the attachment of more superficial cells to the basement
membrane via hemidesmosomal complexes [15,17]. The basal cell is thought to be able to function as a
primary stem cell, giving rise to mucous and ciliated epithelial cells [5,18–25]. Pulmonary endocrine cells
are found throughout the airway as solitary cells or in clusters. These cells secrete a variety of biogenic
amines and peptides, which appear to play an important role in fetal lung development and airway
function including the regulation of epithelial cell growth and regeneration.
The trachea’s rich arterial blood supply is derived from fine branches of the superior and inferior
thyroid arteries, of the internal thoracic arteries, and of the bronchial arteries. Returning blood from
tracheal veins eventually travels into the inferior thyroid veins. The incompletion of the C-shaped rings
allows the trachea to be in close apposition to the esophagus throughout its length and to share vascular
supply. While it does receive its blood supply from named vessels, its vasculature is composed of a rich
network of thin vessels. The profuse system of microvessels that immediately underlie the epithelium is of
particular importance in the maintenance and regeneration of airway epithelium. There is thought to be
a dynamic interplay between plasma-derived molecules, their receptors, airway epithelial cells, and their
secretions in vivo, which either promotes airway defense or induces disease [26].
The smooth muscle and glands of the trachea are parasympathetically innervated by the vagus
nerve, either directly or by the recurrent laryngeal nerves. Sympathetic innervation comes directly from
the sympathetic trunk. Tracheal mucosa itself is richly innervated from subepithelial plexuses. The trachea
is remarkably sensitive to touch and has a low threshold to elicit a reflexive cough in the presence of foreign

mikos: “9026_c033” — 2007/4/9 — 15:53 — page5—#5
Tracheal Tissue Engineering 33-5
material. The subepithelial nerves penetrate the basement membrane at focal points where they branch
and spread along the basement membrane with terminal ends extending between epithelial cells and
terminating in the airway lumen. The nerves have an obvious sensory role but the full breadth of their
exact function is unknown; there is, however, evidence that they might be in direct apposition to pul-
monary endocrine cells, with the suggestion of a bi-directional communication between these two cell
types [5].
In summary, the trachea is a simple, yet elegant, structure that very effectively resists collapse from
negative intraluminal pressures. It is flexible enough to accommodate distension and adapt to cervical
rotation, and is lined with a metabolically active and physiologically complex respiratory epithelium that
is intimately linked to the underlying mesenchyme and the immune system.
33.2 Tracheal Reconstruction: Previous Attempts
Tracheal reconstruction dates back to at least 1881 when Gluck and Zeller re-anastomosed a transected
dog trachea [27]. Over 80 years later in his classic 1964 paper, Grillo described anatomic studies on
human cadavers establishing the upper limits of tracheal resection that would allow a direct end-to-end
anastomosis without undue anastomotic tension [28]. Subsequently, he and others refined the techniques
of tracheal resection with primary anastomosis [29–37]. Today, approximately half of the human adult’s
and one third of the small child’s trachea can safely be resected and primarily anastomosed. Even long
segment tracheal stenosis can now often be handled by an operation known as a slide tracheoplasty.
The need for more extensive resections is clinically rare. In the adult population, the need for replace-
ment of greater than a half of the trachea usually arises in the setting of a low-grade neoplasm, such
as adenoid cystic carcinoma of the trachea, or in the setting of unresectable diseases (such as tracheo-
pathia osteoplastica, relapsing polychondritis, Wegener’s granulomatosis, and trauma). In the neonate,
it arises in the setting of tracheal agenesis, a congenital absence of tracheal tissue [38,39]. Whenever a
significant length of trachea is compromised by disease, it presents a true surgical dilemma as no truly
dependable and reliable replacement yet exists. Given the infrequent clinical demand, it is amazing that
the literature is rich in attempts to find suitable materials with which to replace tracheal tissue. None of
these have been particularly successful and none have found consistent and widely accepted clinical use.
A limited review of some these attempts offers valuable insight into the physiology and pathophysiology
of the trachea. They fall into several categories: implantation of foreign materials, reconstruction with
autogenous tissues, reconstruction, and transplantation of autografts and allografts. The newest category
is tissue engineering [38].
A wide variety of materials has been used for solid prostheses, including but not limited to stainless
steel, Vitallium, glass, polyethylene, Lucite, silicone, Teflon, Ivalon, polyvinyl chloride, and polyurethane
[40–54]. These materials were used as single constituents or in combination; some prostheses used cuffs
draped over tracheal ends to encourage fixation of the prosthesis and prevent obstruction with granulation
tissue at the anastomotic sites. The solid prostheses have been prone to migrate and dislodge, to obstruct
with granulation tissue at the anastomoses, and to develop infections. In addition, they have tended to
yield poor epithelialization. No solid prosthesis has ever proven reliable over time. Some may work for an
unpredictable amount of time but all eventually fail. In response to the failure of solid prostheses, some
groups turned to porous synthetic prostheses to allow tissue ingrowth and promote a greater prosthetic
incorporation. A variety of porous prostheses have been attempted; some of these were used in conjunc-
tion with tissues such as pericardium, omentum, dermis, pleura, and fascia as well as fibrin and collagen
[50,51,55–75]. The porous prostheses have also yielded unsatisfactory results. They have regularly failed
to become fully epithelialized, especially in the center, which promoted central granulation, cicatrisa-
tion (scar formation), and stenosis. The porous prostheses have also shown a propensity for bacterial
colonization.
There have been many attempts to replace trachea with autogenous tissues, including skin, fascia,
pericardium, periosteum, buccal mucosa, aortic tissue, esophageal tissue, bronchial tissue, cartilage,
mikos: “9026_c033” — 2007/4/9 — 15:53 — page6—#6
33-6 Tissue Engineering
bone, and bladder epithelium [38,49,57,58,70,76–92]. The implanted cartilage, despite its autogenous
source, often resorbed. In addition to devascularized autogenous tissues, several authors have reported
using vascularly pedicled tissue transfers. Although some yielded temporary success, none of these have
become commonplace in clinical practice. Some heroic efforts have been documented in the clinical realm.
There are several clinical reports of tracheal reconstruction with cutaneous troughs, as well as esophageal
transfers. These are difficult procedures whose failure rates, not surprisingly, rise with their increasing
complexity.
Experiments using devitalized tissues including cadaveric tracheas have also failed to produce robust
results [93,94]. Cadaveric tracheal grafts have been treated in a variety of ways: irradiation, freeze-
drying, and chemical treatment [58,95–102]. None has resulted in clinically reliable solutions. Devitalized
tracheal tissue is inherently problematic as the cartilage is inevitably doomed to resorption, leading
to tracheomalacia, a degenerative softening of the trachea. The degree of reported epithelialization is
somewhat variable, but it is doubtful that any was truly and thoroughly effective. Our own laboratory
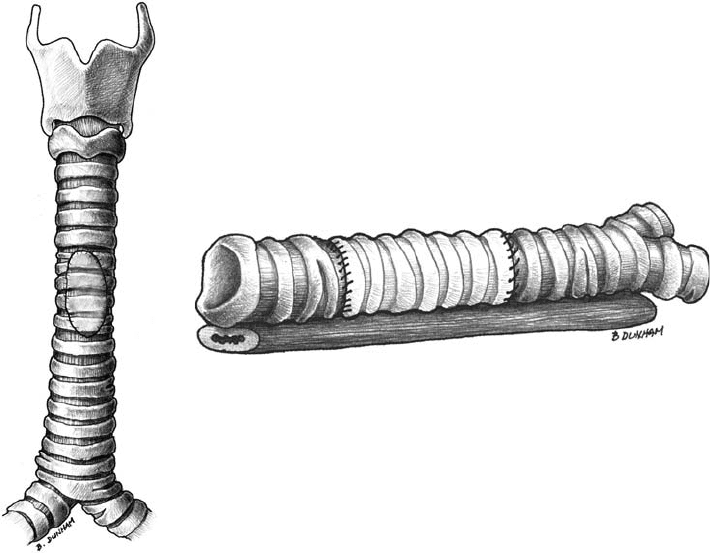
has investigated the use of chemically decellularized cadaveric grafts in a rabbit model, both as anterior
window grafts and circumferential grafts (unpublished data; Figures 33.4a,b). All experimental animals
that underwent an anterior window replacement survived until their appointed time of sacrifice without
suffering from clinically significant stenoses. The circumferential replacement was far more problematic.
All grafts, if given sufficient time, gradually stenosed. Gross evaluation confirmed central malacia of
the grafts, as well as centrally located mucosal stenoses. Histologic evaluation revealed a significant
inflammatory response that showed a predilection for the center of the grafts; epithelial denudation also
appeared to coincide with excessive granulation tissue, as is often reported in the literature.
Early experimental use of true transplants, in other words, fresh tracheal allografts in animal models has
yielded uniformly poor results [57,58,78,99,103]. Even fresh autografts have been problematic. Smaller
(a) (b)
FIGURE 33.4 (a) Schematic of an anterior window replacement. (b) Schematic of a full circumferential tracheal
replacment.
mikos: “9026_c033” — 2007/4/9 — 15:53 — page7—#7
Tracheal Tissue Engineering 33-7
segments and patches tend to have a higher success rate. Fibrous degeneration of the autograft is common.
Presumably, the failure partly occurs because the fine vasculature of the grafts has not reestablished rapidly
enough to support the respiratory mucosa and the underlying mesenchyme before exposure to the external
environment engenders a chronic inflammatory response with devolution of the mucosa. One potential
solution would be the use of vascular anastomoses to allow for near immediate reconnection of a graft’s
vascular network. Unfortunately, although vascular anastomoses are technically feasible as recent work by
Genden [104,105] has shown, they significantly increase the complexity of the surgical procedure and can
increase the risk of failure in the already unforgiving milieu of the airway. Other groups have addressed
this issue by incorporating thyroid tissue along with the trachea during transplantation attempts, using
the thyroid’s larger blood vessels to perform the anastomoses [106,107].
Without immune suppression, fresh allografts have elicited an immune rejection, resulting in ischemic
necrosis of the implanted tissues. These allografts, not surprisingly, suffered from resorptive collapse of
their tracheal rings and poor reepithelization leading to a short postoperative survival of the animals
[57,58,103]. Some improvement in graft viability has occurred with indirect vascularization with omental
flaps. This has been especially true for small grafts; longer grafts, however, still have not fared well. Allo-
grafts that have undergone cryopreservation and have been supported with an omental flap have shown
improved survival, even without immunosuppression[108–110]. However, recipient chondrocytes have
not repopulated the grafts’ cartilage. Cryopreservation, presumably, has reduced epithelial antigenicity;
this phenomenon is not yet fully understood. Bujia [111] showed evidence that the predominant locus
of antigenicity is likely to reside in the epithelium and not the cartilage. Liu denuded tracheal epithelium
with detergent and reported that these allografts, which were supported with an omental flap, remained
viable [112,113]. Not surprisingly, given airway mucosa regenerative capacity, recipient epithelial cells
have eventually repopulated the grafts’ epithelium.
In an effort to induce immunotolerance, Genden and colleagues pretreated rats with a single portal-vein
injection of ultraviolet-B irradiated donor splenocytes seven days prior to circumferential tracheal allo-
graft placement. The pretreatment induced a donor-specific immune hyporesponsiveness and prevented
rejection of the grafts [114]. Whether or not immune tolerance can be induced in higher animal models
remains to be seen.
Tracheal obstruction is seen time and time again in tracheal reconstruction efforts. Provided that a
neotrachea would be able to resist collapse, it still faces a tremendous challenge within its lumen: that
of establishing a healthy mucosa, free of chronic inflammation. The luminal compromise is consistently
associated with a detrimental soft tissue reaction, in which airway mucosa undergoes a significant reactive
thickening with a progressive diminution of the airway lumen and a disruption of the structural integrity
of the cartilaginous rings of the trachea. This reaction appears to be superficially analogous to exuberant
scarring found in skin tissue. It is thought that prompt and thorough reepithelialization can prevent this
complication and maintain luminal patency [115].
Our present ability to intelligently address the infrequent need for tracheal tissue replacement is
hampered by our lack of understanding airway mucosal of wound healing. Significant strides have been
made in the tissue engineering of cartilage; the ability to engineer a structure that is able to resist col-
lapse and that meets the rigors of clinical application is likely to be imminent. However, a great deal
remains to be understood about airway mucosal physiology and healing before true clinical utility will be
possible.
Although the need for full circumferential tracheal replacement is limited, the need to address airway
mucosal disease is far greater. The incidence of tracheal stenosis after an ischemic mucosal injury is far
more prevalent than those etiologies requiring replacement of more than half of the trachea. Since the
advent of cuffed endotracheal tubes, the incidence of tracheal stenosis has risen sharply. The presence of
a foreign body within the airway, especially one that applies mucosal pressure denuding epithelium and
compromising blood flow, can trigger a very poorly understood chronic mucosal inflammation, which
often results in mucosal hypertrophy and luminal obstruction. The problem is compounded by the fact
that the airway is constantly exposed to the bacteria-laden external environment. So, whereas the literature
seems to have focused on circumferential tracheal tissue replacement, clinically there is a far greater need
