Blake A.J.(ed.) Crystal Structure Analysis
Подождите немного. Документ загружается.

22 Introduction to symmetry and diffraction
shape, but some trigonal crystal structures have a different (rhombohe-
dral) lattice not discussed here in detail, so there are just seven possible
different metric symmetries.
Some properties of the 32 crystallographic point groups are summa-
rized in Table 2.5. Here, in the last column, an enantiomorphous point
Table 2.5. The 32 crystallographic point groups.
symm along
system,
Laue, centring
point
group
space
group nos. xyz order E/P/C
Triclinic 11 111 1EP
1 P 12 1 1 12C
monoclinic 2 3–5 1 2 1 2 E P
2/mPC m 6–9 1 m 12P
2/m 10–15
12/m 14C
orthorhombic 222 16–24 2 2 2 4 E
mmm P C I F mm2 25–46 mm24P
mmm 47–74 2/m 2/m 2/m 8C
z x,y xy
tetragonal 4 75–80 4 1 1 4 E P
4/mPI
4 81–82 411 4
4/m 83–88 4/m
1 18C
tetragonal 422 89–98 4 2 2 8 E
4/mmm P I 4mm 99–110 4 mm 8P
42m 111–122 42m 8
4m2 4 m 2
4/mmm 123–142 4/m 2/m 2/m 16 C
trigonal 3 143–146 3 1 1 3 E P
3 PR 3 147–148 3 1 16C
trigonal 321 149–155 3 2 1 6 E
3mPR 312 3 1 2
3m1 156–161 3 m 16P
31m 31m
3m1 162–167 32/m 112C
31m 3 12/m
hexagonal 6 168–173 6 1 1 6 E P
6/mP
6 174 611 6
6/m 175–176 6/m
1 112C
hexagonal 622 177–182 6 2 2 12 E
6/mmm P 6mm 183–186 6 mm 12 P
62m 187–190 62m 12
6m2 6 m 2
6/mmm 191–194 6/m 2/m 2/m 24 C
x,y,z xyz xy,yz,zx
cubic 23 195–199 2 3 1 12 E
m
3 PIF m3 200–206 2/m 3 124C
cubic 432 207–214 4 3 2 24 E
m
3mPIF 43m 215–220 43m 24
m
3m 221–230 4/m 32/m 48 C
2.6 Further points 23
group (E) means one available for the crystallization of optically pure
chiral molecules; a polar point group (P) is one in which at least one par-
ticular direction is symmetrically distinct from the opposite direction;
C means a centrosymmetric point group. The order of a point group is
the number of equivalent general positions in each of the related space
groups with P unit cells, and must be multiplied by 2 for A, C and I,by
3 for R, and by 4 for F cells. For some tetragonal, trigonal and hexagonal
point groups, differentarrangements of symmetry elements are possible
for the a- and b-axes and the directions lying between them, leading to
the use of alternative symbols so that different but related space groups
are clearly distinguished; these are shown in adjacent rows.

24 Introduction to symmetry and diffraction
Exercises
1. You are provided in Fig. 2.3 and Fig. 2.4 with four two-
dimensional repeating patterns (Traidcraft gift wrap-
ping paper!). For each one, identify lattice points and
outline a unit cell (possible shapes are oblique, rectan-
gular, square, and hexagonal; a rectangular unit cell can
be primitive or centred). Find the symmetry elements;
for a 2D pattern the following are possible: 2-, 3-, 4- and
6-fold rotations, mirror lines, and glide lines (mirrors
with a half-unit-cell translation component parallel to
the reflection line); in 2D inversion symmetry is the same
as a 2-fold rotation. Show what fraction of the unit cell
is the asymmetric unit.
(1)
(2)
Fig. 2.3 Patterns for Exercise 1.
(3)
(4)
Fig. 2.4 Patterns for Exercise 1.
2. The point group of a ferrocene molecule [Fe(C
5
H
5
)
2
]
is D
5h
, assuming an eclipsed conformation of the two
rings. This point group symmetry is not possible in the
crystalline solid state (no 5-fold rotation axes!). The sym-
metry elements of D
5h
are: a five-fold rotation axis, 5
two-fold rotation axes perpendicular to this, 5 ‘verti-
cal’ mirror planes each containing Fe and 2 C atoms,
and one ‘horizontal’ mirror plane through Fe and lying
between the two rings (there is also an S
5
improper rota-
tion axis). Which of these symmetry elements could be
retained in the site symmetry of a ferrocene molecule in
a crystal structure,and what is the highest possible point

Exercises 25
group symmetry for ferrocene in the crystal (the maxi-
mum number of symmetry elements that can be retained
simultaneously)?
3. Why does the list of conventional Bravais lattices not
include any centred unit cells in the triclinic system,
tetragonal C , or cubic C?
4. Work out the point group andthe Laue class correspond-
ing to the following space groups: (a) C2; (b) Pna2
1
; (c)
Fd
3c; (d) I4
1
cd.
5. From the space group symbols alone, what (if any) spe-
cial positions would you expect to find for (a) P
1; (b) C2;
(c) P2
1
2
1
2
1
?
This page intentionally left blank

3
Crystal growth and
evaluation
Alexander Blake
3.1 Introduction
Whether growing crystals or giving advice to someone else trying to
do so, it is vital to remember that the quality of the crystal from which
diffraction data are acquired is generally the main determinant of the
final quality of the structure. The effects of a suboptimal crystal will
propagate through data collection, structure solution and refinement to
affect the quality of the final structure, in which unsatisfactorily high
uncertainties may limit useful comparison and discussion. It may be
difficult or impossible to get such a structure published.
3.2 Protect your crystals
Before you start trying to grow crystals you need to think about how
they will be handled. They will need to be extracted from the vessel they
grew in without suffering any damage. Less obviously, some containers
make this easier than others: an oversized one like a 250-ml round-
bottomed flask makes the procedure of finding and removing a small
crystal unnecessarily difficult. At the other extreme, a container with a
small aperture that will not admit a narrow spatula or pipette is also
troublesome. You should avoid screw-top or other containers that nar-
row near the top, as the ‘shoulders’ prevent easy removal of the crystals:
a small vial with straight walls works best.
Many crystals lose solvent on removal from the solution in which
they have grown (mother liquor). Although the envelope of the crys-
tals may appear intact, even a few per cent solvent loss usually renders
them useless for structural analysis. This is particularly common for
crystals grown from chlorocarbon solvents like dichloromethane and
chloroform,but can affect crystals grown from almost any solvent,water
included. If a crystal loses solvent and then does not behave well on
the diffractometer, there is no way to know whether the original crys-
tal was unsuitable, or whether the poor diffraction was due solely to
27
28 Crystal growth and evaluation
solvent loss. For this reason you should keep crystals under mother
liquor whenever possible. There are other good reasons for doing this –
see below.
3.3 Crystal growth
The term recrystallization has two related meanings but thereare crucial
differences between these. In the synthesis and purification of compounds,
the aim is to maximize purity and yield, although these can be mutually
exclusive.The material is oftenprecipitatedveryrapidly(∼1 s), resulting
in microcrystalline or virtually amorphous products that are useless
for conventional single-crystal work. For diffraction work the object is to
obtain a small number (one may do) of relatively large (∼0.1–0.4 mm)
singlecrystals.Aslongasthis is achieved, yield is irrelevantandpurityis
likelyto be enhanced.Tothisend, crystals shouldbegrown slowly,taking
from minutes to months depending on the system. To understand why
this is important, visualize the process of growth at a crystal surface. The
greater the rate at which molecules arrive at the surface, the less time
they have to orient themselves in relation to molecules already there:
random accretion is more likely, leading to crystals that are twinned or
disordered. Suitable growth conditions include the absence of dust and
vibration: if these are present they can lead to small or non-singular
crystals.
3.4 Survey of methods
3.4.1 Solution methods
These are by far the most flexible and widely used. They are suitable for
use with molecular compounds that are the subject of most crystal struc-
ture determinations. The use of solvents means that crystals can grow
separately from each other. It is therefore important not to let a solu-
tion dry out, as crystals could become encrusted and may not remain
single. When choosing solvents remember the general rule that ‘like dis-
solves like’: look for a solvent that is similar to the compound (in terms
of polarity, functional groups, etc.) and an antisolvent that is dissimi-
lar to it in order to reduce its solubility (see below). Information about
solvents is available from several sources (e.g. Handbook of Physics and
Chemistry, chromatographic elution data), and the process of synthesiz-
ing and purifying a compound will often confer a knowledge of suitable
solvents. Mixing solvents allows manipulation of solubility: a mixture
of solvent A (in which a compound is too soluble) and antisolvent B
(in which it is not sufficiently soluble) may be more useful than either
alone. If crystals grown from one solvent are poor in quality, try differ-
ent solvents or mixtures of solvents. Solution methods can be extremely
flexible: a number of crystallizations, differing in the proportions of sol-
vents A and B used, can be set up to run in parallel. If a particular range
3.4 Survey of methods 29
of proportions appears to be more successful in producing crystals it
can be investigated more closely by decreasing the difference between
successive mixtures of A and B.
It is important that any vessels used for crystal growth should be
free of contaminants. Older containers also tend to have a large number
of scratches and other surface defects, providing multiple nucleation
points and tending to give large numbers of small crystals. Two factors
that favour the formation of twinned crystals are the presence of impu-
rities and uneven thermal gradients. Conversely, if the inner surface of
a container is too smooth this may inhibit crystallization. If this appears
to be the case, gently scratching the surface with a metal spatula a few
times may be effective. Some of the possible variations are described
briefly below, and virtually all methods described can be adapted to
accommodate air sensitivity.
Concentration. If the volume of a solution is reduced, for example
by evaporation of a volatile solvent, the concentration of the solute will
rise until it begins to crystallize. When using mixed solvents the poorer
solvent should be the less volatile so that the solubility of the solute
decreases upon evaporation (but see Fig. 3.1). The rate of evaporation
can be controlled in various ways, for example by altering the tempera-
ture of the sample or by adjusting the size of the aperture through which
the solvent vapour can escape. As solvents are frequently flammable or
irritants, it is important to work on the smallest scale possible and ensure
than any vapour released from the solution is safely dealt with. Avoid
obvious hazards such as those that will arise if large volumes of diethyl
ether or other highly volatile solvents are allowed to evaporate in a
closed container such as a refrigerator.
As noted above, it is highly undesirable to let a solution evaporate
to dryness as this will allow otherwise suitable crystals to become
encrusted,grow into an aggregateor be contaminated by impurities. The
crystals may be degraded by loss of solvent of crystallization, especially
ifchlorocarbonsolventssuchasdichloromethane havebeenused.It may
prove impossible to identify good crystals even if these are present, and
extracting them undamaged from a mass of material may prove difficult
or impossible.
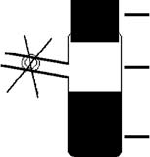
Large rubber Subaseal
Small Schlenk tube
Solution in a mixture
of solvents
Fig. 3.1 A method of controlling solubility
by selectively removing the less volatile sol-
vent in which the compound is more solu-
ble. This (chlorocarbon) solvent is absorbed
by the rubber Subaseal, while the antisol-
vent (diethyl ether) is not, leading to a
more concentrated solution and eventually
to crystallization.
Apparently sealed NMR tubes that have been forgotten at the back of
a fume cupboard or fridge for weeks or months are a fruitful source of
good-quality crystals: there is in fact slow evaporation of solvent and
crystals are able to grow undisturbed. As long as the NMR tube is clean
and relatively unscratched the smooth inner surface and narrow bore
provide an excellent environment for crystal growth.
Cooling. Either make up a hot, nearly saturated solution and allow
it to cool slowly towards room temperature or make up such a solu-
tion at room temperature and cool it slowly in a fridge or freezer. The
cooling rate can be reduced by exploiting the fact that the larger and
more massive an object, the longer it will take to lose heat. Thus, a hot
solution in a large vessel (or in a small vessel within a larger one) will
cool relatively slowly (Fig. 3.2, left). Similarly, a sample tube containing
30 Crystal growth and evaluation
Capped vial
Cooling
Solution
Crystals
collect here
Heat
Heat
Sample
Crystals
Saturated
solution
Thermal
reservoir
Fig. 3.2 Controlled cooling (left) and an apparatus to exploit convection (right).
a solution will cool at a slower rate if it is contained in a metal block that
was originally at room temperature, or if surrounded by an effective
layer of insulation. Cooling methods are based on the generally valid
assumption that solubility decreases with temperature. There are rare
exceptions to this (e.g. Na
2
SO
4
in water) and some solubilities rise so
rapidly with temperature that it can be difficult to control crystalliza-
tion (e.g. of KNO
3
from water). However, it is usually possible to find
a combination of solute and solvent where solubility varies slowly and
controllably with temperature.
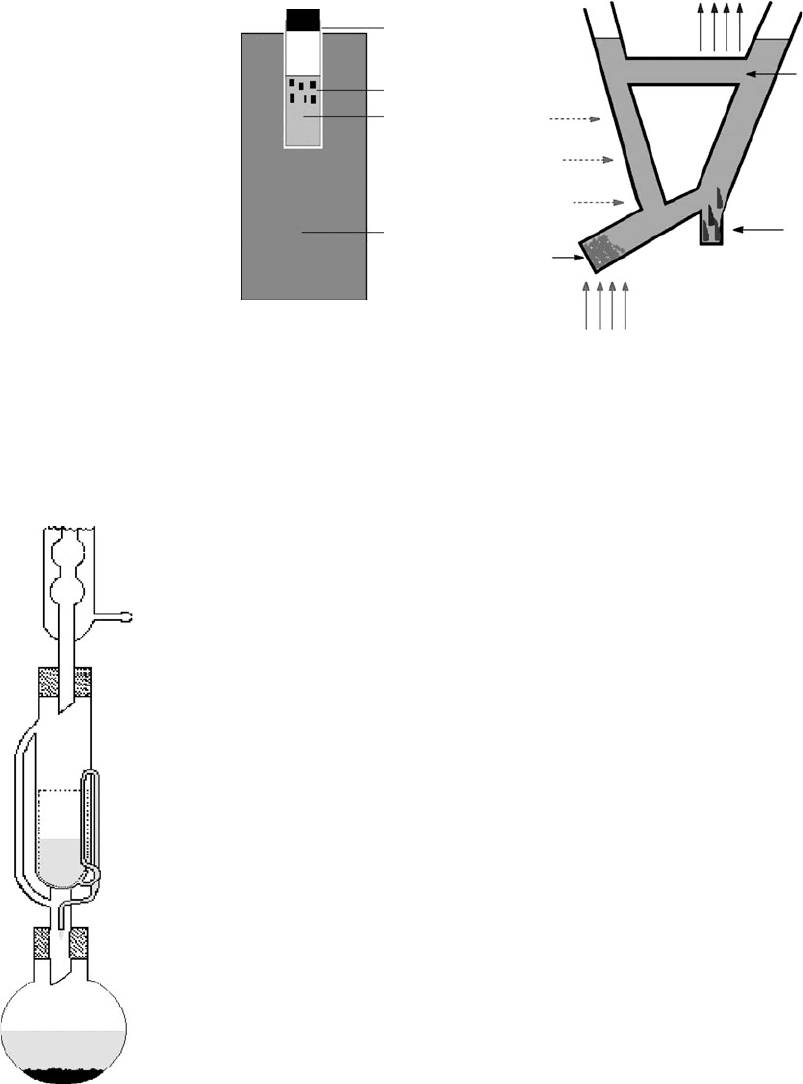
Fig. 3.3 Soxhlet apparatus.
Convection. The aim hereis to establish a temperaturegradient across
the solution, so that material dissolves in the warmer area and deposits
in the colder. This gradient can be established by various means, for
example (a) allow sunlight to shine on one part of the vessel; (b) put one
part of the vessel against a cooler surface, such as a window at night;
(c) construct an apparatus with low-power electrical heating elements
in some sections (Fig. 3.2 right). A smooth concentration gradient will
give the best results.
One method that combines variation of both concentration and tem-
perature and is especially useful for sparingly soluble compounds is
Soxhlet extraction (Fig. 3.3). The recycling of the solvent is the key fac-
tor here and crystals can even appear in the refluxing solvent. Failing
this, they normally appear after slow cooling of the solution. There are
several other methods available to control concentration. One of these
is based on osmosis, where the solvent passes through a semipermeable
membrane into a concentrated solution of an inert species. The resulting
increase in the concentration of the solute may lead to crystal formation.
Solvent diffusion. This method is based on the fact that a compound
will dissolve well in certain solvents (‘good solvents’) but not in others
(‘poor solvents’ or antisolvents), which must be co-miscible. Dissolve

3.4 Survey of methods 31
the compound in the ‘good solvent’ and place this solution in a narrow
tube. Using a syringe fitted with a fine needle, very slowly inject the
neat antisolvent. If it is lighter than the solution, layer it on top; if it is
denser, inject it slowly into the bottom of the tube to form a layer under
the solution. Injecting the solvent is better than running it down the
side of the tube. If the tube is protected from vibration, these layers will
mix slowly and crystals will grow at the interface (Fig. 3.4). If necessary,
cooling of the tube can be used both to lower the rate of diffusion and
to reduce the solubility.
Fig. 3.4 Solvent diffusion.
Gaps
Inner
vessel
Anti-
solvent
Solution
Outer
vessel
(closed)
Fig. 3.5 Vapour diffusion.
Vapour diffusion. This method is also called isothermal distillation.
The antisolvent diffuses through the vapour phase into a solution of
the compound in the ‘good solvent’, thereby reducing the solubility
(Fig. 3.5). The advantages of this method include the relatively slow
rate of diffusion, its controllability and its adaptability, for example in
combination with Schlenk techniques to grow crystals of air-sensitive
samples. It is usually worth trying vapour diffusion as it frequently
succeeds where other methods have failed. A variant on this is the
hanging-drop method, principally used for the growth of crystals of
proteins and other macromolecules: the precipitant sits in a well and dif-
fuses slowly into a drop of solution suspended on a glass slide covering
the well.
If you are using a needle to make holes in the polythene cap of a
sample vial, take particular care to remove completely any slugs of poly-
thene formed by this procedure. They may be inside the vial or hanging
loosely from the cap. If these slugs get into your sample they can look
remarkably like large single crystals (Fig. 3.6), and even show plausible
optical properties due to the stress of their formation. They can often be
identified by the presence of a tail-like extension, but this is not always
present, and they are often identified only by their diffraction pattern
(Fig. 3.7). Making holes from the inside of the cap outwards makes it
easier to check for the presence of slugs, but close scrutiny of vial and
cap is always worthwhile.
Reactant diffusion. It is sometimes possible to combine synthesis and
crystal growth. In favourable cases crystals may simply drop out of the
reaction mixture, but the rate of many reactions means that crystals form
rapidly and are therefore small and of low quality. If the reaction rate
Fig. 3.6 A polythene slug produced by using a needle to make holes in the cap of a vial.
