Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

1.4
Use of the Equations of Change to Solve Steady-State Problems
347
SOLUTION
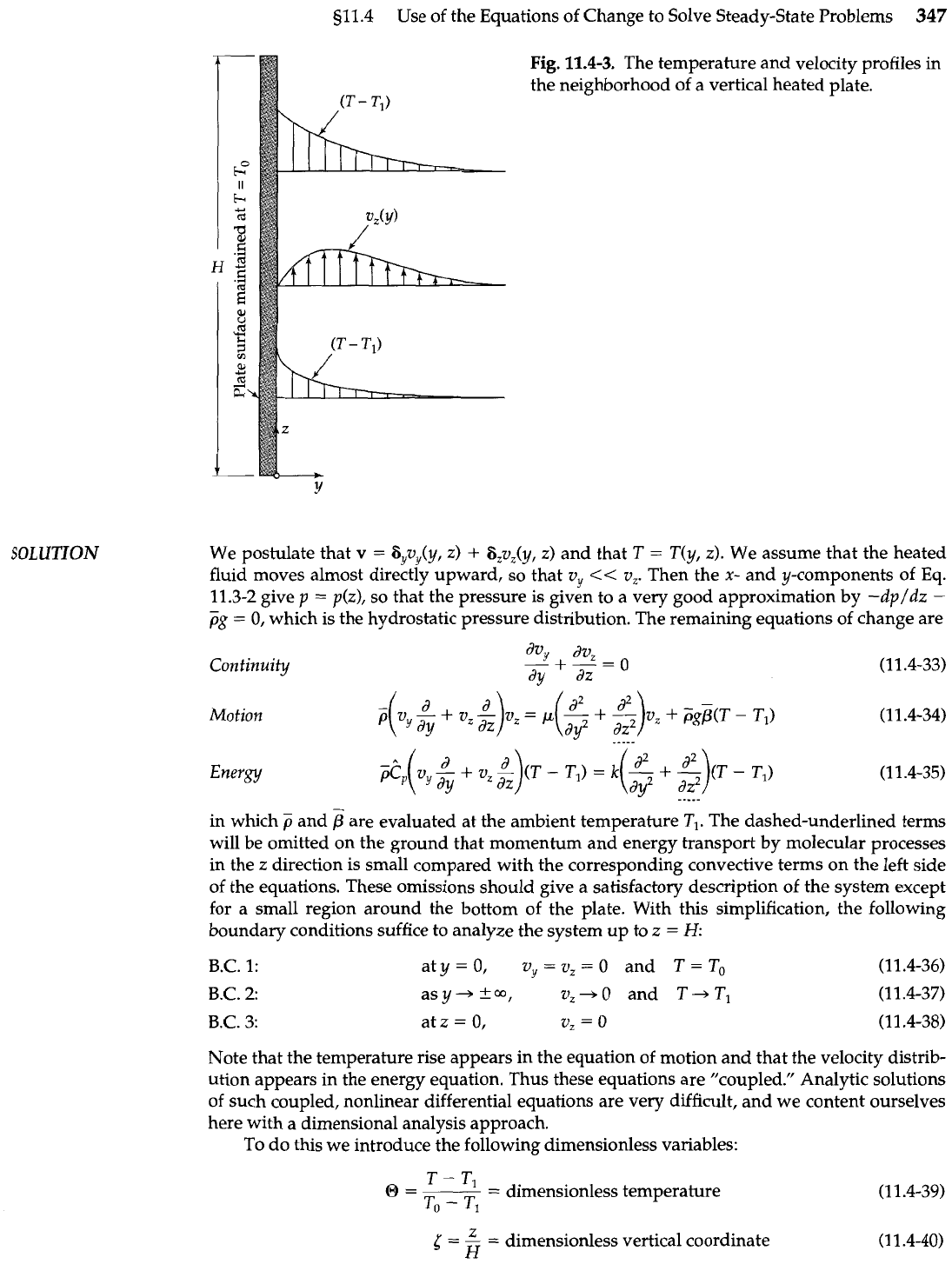
Fig.
11.4-3.
The temperature and velocity profiles
the neighborhood of a vertical heated plate.
We postulate that
v
=
6,v,(y,
z)
+
6,v,(y, z) and that
T
=
T(y, 2). We assume that the heated
fluid moves almost directly upward, so that v,
<<
v,. Then the
x-
and y-components of Eq.
11.3-2 give
p
=
p(z), so that the pressure is given to a very good approximation by
-dp/dz
-
pg
=
0, which is the hydrostatic pressure distribution. The remaining equations of change are
Continuity
Motion
Energy
-----
in which
p
and
p
are evaluated at the ambient temperature TI. The dashed-underlined terms
will be omitted on the ground that momentum and energy transport by molecular processes
in the
z
direction is small compared with the corresponding convective terms on the left side
of the equations. These omissions should give a satisfactory description of the system except
for a small region around the bottom of the plate. With this simplification, the following
boundary conditions suffice to analyze the system up to
z
=
H:
B.C.
1:
B.C.
2:
B.C.
3:
aty=O, v,=v,=O and
T=T,
asy+
?m,
v,+O
and T+T1
at
z
=
0,
v,
=
0
Note that the temperature rise appears in the equation of motion and that the velocity distrib-
ution appears in the energy equation. Thus these equations are "coupled." Analytic solutions
of such coupled, nonlinear differential equations are very difficult, and we content ourselves
here with a dimensional analysis approach.
To do this we introduce the following dimensionless variables:
T
-
TI
0
=
---
=
dimensionless temperature
To
-
TI
2
5
=
-
=
dimensionless vertical coordinate
H
(1
1
.4-40)
348
Chapter
11
The Equations of Change for Nonisothermal Systems
1
/4
,r,
=
(&)
y
=
dimensionless horizontal coordinate
9,
=
(--&)1'2uz
=
dimensionless vertical velocity
(1
1.4-42)
pH
dy
=
(z)
vy
=
dimensionless horizontal velocity
(1
1.4-43)
in which
a
=
k/&
and
B
=
F~~(T~
-
TI).
When the equations of change, without the dashed-underlined terms, are written
in
terms of these dimensionless variables, we get
Continuity
Motion
Energy
The preceding boundary conditions then become
B.C.
1:
B.C.
2:
B.C.
3:
One can see immediately from these equations and boundary conditions that the dimension-
less velocity components
$
and
4,
and the dimensionless temperature
@
will depend on
7
and
l
and also on the Prandtl number, Pr. Since the flow is usually very slow in free convec-
tion, the terms in which Pr appears will generally be rather small; setting them equal to
zero
would correspond to the "creeping flow assumption." Hence we expect that the dependence
of the solution on the Prandtl number will be weak.
The average heat
flux
from one side of the plate may be written as
The integral may now be written in terms of the dimensionless quantities
in which the grouping Ra
=
GrPr is referred to as the
Rayleigk number.
Because
O
is a function
of
77,
l,
and Pr, the derivative
dO/dv
is also a function of
,r,,
l,
and Pr. Then
dO/dq,
evaluated
at
,r,
=
0, depends only on
l
and Pr. The definite integral over
5
is
thus a function of Pr. From
the remarks made earlier, we can infer that this function, called
C,
will be only a weak func-
tion of the Prandtl number-that is, nearly a constant.
The preceding analysis shows that, even without solving the partial differential equa-
tions, we can predict that the average heat flux is proportional to the $-power of the tempera-
ture difference
(To
-
TI)
and inversely proportional to the +-power of
H.
Both predictions
have been confirmed by experiment. The only thing we could not do was to find
C
as a func-
tion of Pr.

g11.4
Use of the Equations of Change to Solve Steady-State Problems
349
Adiabatic Frictionless
Processes in
an
Ideal
Gas
To determine that function, we have to make experimental measurements or solve Eqs.
11.4-44 to 49. In 1881, Lorenz3 obtained an approximate solution to these equations and found
C
=
0.548. Later, more refined calculations4 gave the following dependence of
C
on Pr:
Pr 0.73 (air)
1
10
100 1000
C
0.518 0.535
0.620 0.653 0.665 0.670
These values of
C
are nearly in exact agreement with the best experimental measurements in
the laminar flow range (i.e., for GrPr
<
lo9h5
Develop equations for the relationship of local pressure to density or temperature in a stream
of ideal gas in which the momentum flux
.r
and the heat flux
q
are negligible.
SOLUTION
With
7
and
q
neglected, the equation of energy [Eq.
(1)
in Table 11.4-11 may be rewritten as
For an ideal gas,
=
RT/M, where M is the molecular weight of the gas, and Eq. 11.4-52
becomes
Dividing this equation by
p
and assuming the molar heat capacity
$
=
MS
to be constant,
we can again use the ideal gas law to get
Hence the quantity in parentheses is a constant along the path of a fluid element, as is its an-
tilogarithm, so that we have
TC,/R
1
=
p
constant
(1
1.4-55)
This relation applies to all thermodynamic states
p,
T
that a fluid element encounters as it
moves along with the fluid.
Introducing the definition
y
=
tp/Pv
and the ideal gas relations
$
-
Sv
=
R and
p
=
pRT/M, one obtains the related expressions
p'y-l"y~-'
=
constant
(1
1.4-56)
and
pp-Y
=
constant
(1
1.4-57)
These last three equations find frequent use in the study of frictionless adiabatic processes in
ideal gas dynamics. Equation 11.4-57 is
a
famous relation well worth remembering.
L. Lorenz,
Wiedemann's Ann. der Physik u. Chemie,
13,42247,582406 (1881). See also
U.
Grigull,
Die Grundgesetze der Warrneiibertragung,
Springer-Verlag, Berlin, 3rd edition (1955), pp. 263-269.
%ee
S.
Whitaker,
Fundamental Principles
of
Heat Transfer,
Krieger, Malabar Fla. (1977), g5.11. The
limiting case of Pr
+
w
has been worked out numerically by E.
J.
LeFevre [Heat Div. Paper 113, Dept.
Sci. and Ind. Res., Mech. Engr. Lab. (Great Britain), Aug. 19561 and it was found that
Equation 11.4-51a corresponds to the value
C
=
0.670 above.
This
result has been verified experimentally
by
C.
R.
Wilke,
C.
W. Tobias, and
M.
Eisenberg,
J.
Electrochem. Soc.,
100,513-523 (1953), for the analogous
mass transfer problem.
For
an
analysis of free convection in three-dimensional creeping flow, see W.
E.
Stewart,
Int.
J.
Heat
and Mass Transfer,
14,1013-1031 (1971).
350
Chapter 11 The Equations of Change for Nonisothermal Systems
EXAMPLE
11.47
One-Dimensional
Compressible Flow:
Velocity, Temperature,
and Pressure Profiles
in
a
Stationa
y
Shock
Wave
When the momentum flux
T
and the heat flux
q
are zero, there is no change in entropy
following an element of fluid (see Eq. 11D.1-3). Hence the derivative d In p/d In
T
=
y/(y
-
1)
following the fluid motion has to be understood to mean
(d
In p/d In
T)s
=
y/(y
-
1).
This
equation is a standard formula from equilibrium thermodynamics.
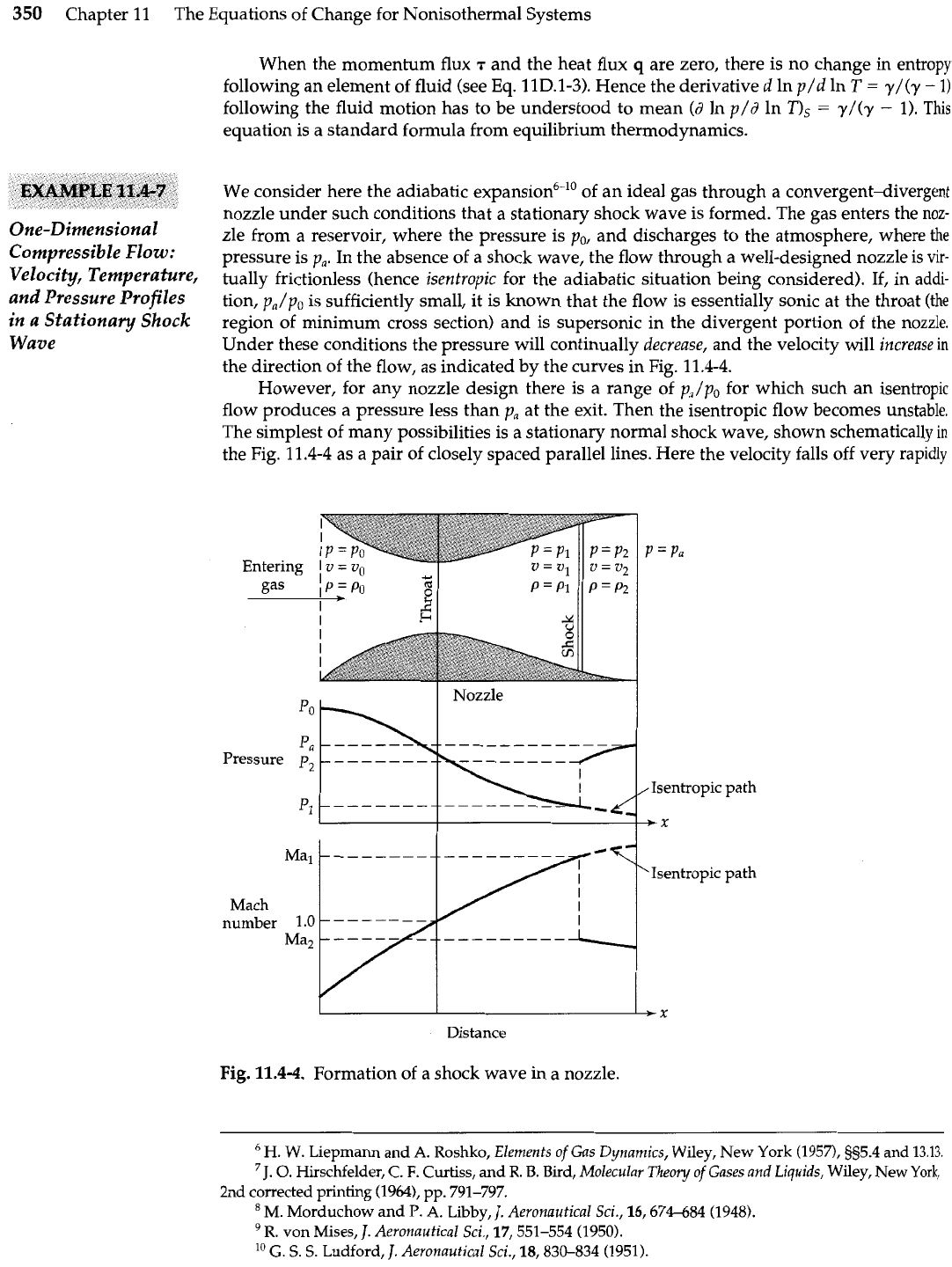
We consider here the adiabatic expansion6-lo of an ideal gas through a convergent-divergent
nozzle under such conditions that a stationary shock wave is formed. The gas enters the noz-
zle from a reservoir, where the pressure is po, and discharges to the atmosphere, where
the
pressure is p,. In the absence of a shock wave, the flow through a well-designed nozzle is vir-
tually frictionless (hence isentropic for the adiabatic situation being considered).
If,
in addi-
tion, p,/po is sufficiently small, it is known that the flow is essentially sonic at the throat
(the
region of minimum cross section) and is supersonic in the divergent portion of the nozzle.
Under these conditions the pressure will continually decrease, and the velocity will increase
in
the direction of the flow, as indicated by the curves in Fig. 11.4-4.
However, for any nozzle design there is a range of p,/po for which such an isentropic
flow produces a pressure less than p, at the exit. Then the isentropic flow becomes unstable.
The simplest of many possibilities is
a
stationary normal shock wave, shown schematically
in
the Fig. 11.4-4 as a pair of closely spaced parallel lines. Here the velocity falls off very rapidly
I
gas
I
P
=
PO
It
I
Nozzle
Mach
Distance
Fig.
11.4-4.
Formation of a shock wave in a nozzle.
.
Isentropic path
+X
'
Isentropic path
'
H.
W.
Liepmann and
A.
Roshko,
Elements of Gas Dynamics,
Wiley, New York (1957), 995.4 and 13.13.
J.
0.
Hirschfelder,
C.
F.
Curtiss, and
R.
B.
Bird,
Molecular Theory of Gases and Liquids,
Wiley, New
York,
2nd corrected printing (1964), pp. 791-797.
M. Morduchow and
P.
A.
Libby,
J.
Aeronautical Sci.,
16,674-684 (1948).
R.
von Mises,
J.
Aeronautical Sci.,
17,551-554 (1950).
'O
G.
S.
S.
Ludford,
J.
Aeronautical Sci.,
18,830-834 (1951).

511.4 Use of the Equations of Change to Solve Steady-State Problems
351
SOLUTION
to a subsonic value, while 60th the pressure and the density rise. These changes take place in
an extremely thin region, which may therefore be considered locally one-dimensional and
laminar, and they are accompanied by a very substantial dissipation of mechanical energy.
Viscous dissipation and heat conduction effects are thus concentrated in an extremely small
region of the nozzle, and it is the purpose of the example to explore the fluid behavior there.
For simplicity the shock wave will be considered normal to the fluid streamlines; in practice,
much more complicated shapes are often observed. The velocity, pressure, and temperature
just upstream of the shock can be calculated and will be considered as known for the pur-
poses of this example.
Use the three equations of change to determine the conditions under which a shock wave
is possible and to find the velocity, temperature, and pressure distributions in such a shock
wave. Assume steady, one-dimensjonal flow of an ideal gas, neglect the dilatational viscosity
K,
and ignore changes of p,
k,
and
Cp
with temperature and pressure.
The equations of change in the neighborhood of the stationary shock wave may be simpli-
fied to
Continuity:
d
;s;
PV,
=
0
(1
1.4-58)
Motion:
Energy:
The energy equation is in the form of Eq.
J
of Table 11.4-1, written for an ideal gas in a steady-
state situation.
The equation of
continuity
may be integrated to give
in which
p1
and
v1
are quantities evaluated a short distance upstream from the shock.
In the
energy
equation we eliminate
pv,
by use of Eq. 11.4-61 and
dp/dx
by using the
equation of motion to get (after some rearrangement)
We next move the second term on the right side over to the left side and divide the entire
equation by
p,v,.
Then each term is integrated with respect to
x
to give
A
in which
C,
is a constant of integration and Pr
=
C,p/k.
For most gases Pr is between 0.65 and
0.85, with an average value close to 0.75. Therefore, to simplify the problem we set Pr equal to
3
z.
Then Eq. 11.4-63 becomes a first-order, linear ordinary differential equation, for which the
solution is
Since
t,T
+
iv;
cannot increase without limit in the positive
x
direction, the second integra-
tion constant
CI1
must be zero. The first integration constant is evaluated from the upstream
conditions, so that
Of course, if we had not chosen Pr to be
2,
a numerical integration of Eq. 11.4-63 would have
been required.
352
Chapter 11 The Equations of Change for Nonisothermal Systems
Next we substitute the integrated continuity equation into the equation of
motion
and in-
tegrate once to obtain
Evaluation of the constant
CIIl
from upstream conditions, where dv,/dx
=
0,
gives
CIII
=
plv:
+
pl
=
plIv:
+
(RTl/M)I. We now multiply both sides by vx and divide by plvl. Then,
with the help of the ideal gas law,
p
=
pRT/M, and Eqs. 11.4-61 and 65, we may eliminate
p
from Eq. 11.4-60 to obtain a relation containing only v, and
x
as variables:
This equation can, after considerable rearrangement, be rewritten in terms of dimensionless
variables:
The relevant dimensionless quantities are
vx
4
=
-
=
dimensionless velocity
Vl
(1
1.4-69)
X
5
=
-
=
dimensionless coordinate
h
(1
1.4-70)
Ma
-
Vl
=
Mach number at the upstream condition
I--
(1 1.4-71)
The reference length
h
is the mean free path defined in Eq. 1.4-3 (with
d2
eliminated by use
of
Eq. 1.4-9):
We may integrate Eq. 11.4-68 to obtain
-
'
=
explPMa1(l
-
a)((
-
&)I
(a
<
4
<
1)
(4
-
a)"
This equation describes the dimensionless velocity distribution
4(5)
containing an integration
constant
to
=
x,/h, which specifies the position of the shock wave in the nozzle; here
to
is con-
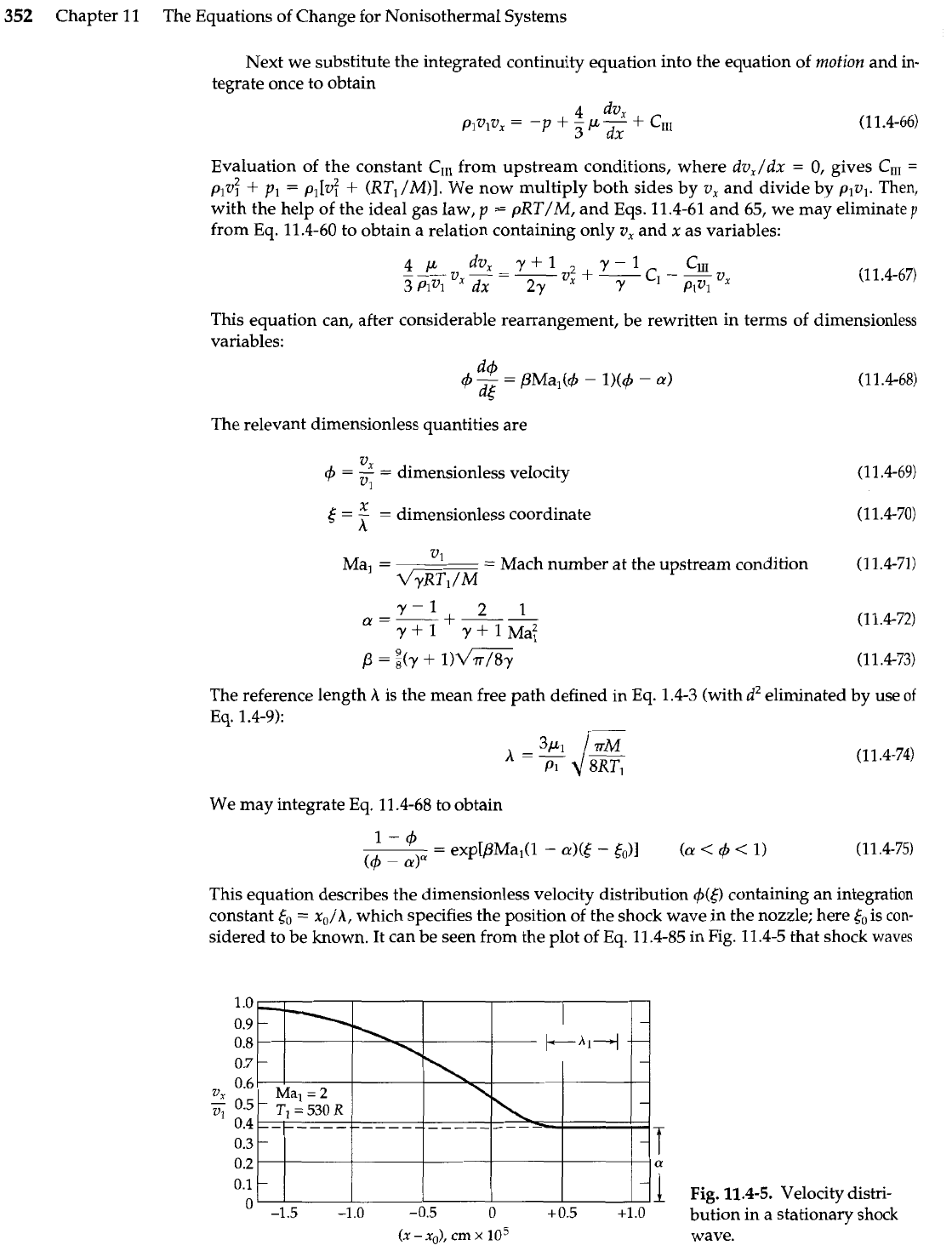
sidered to be known. It can be seen from the plot of Eq. 11.4-85 in Fig. 11.4-5 that shock
waves
Fig.
11.4-5.
Velocity distri-
bution in
a
stationary shock
(X
-
xo),
cm
x
lo5
wave.
911.5
Dimensional Analysis of the Equations of Change for Nonisothermal Systems
353
o
0.00025 in. wire
A
0.002
in.
wire
Theory, equations of
Dimensionless position in flow direction
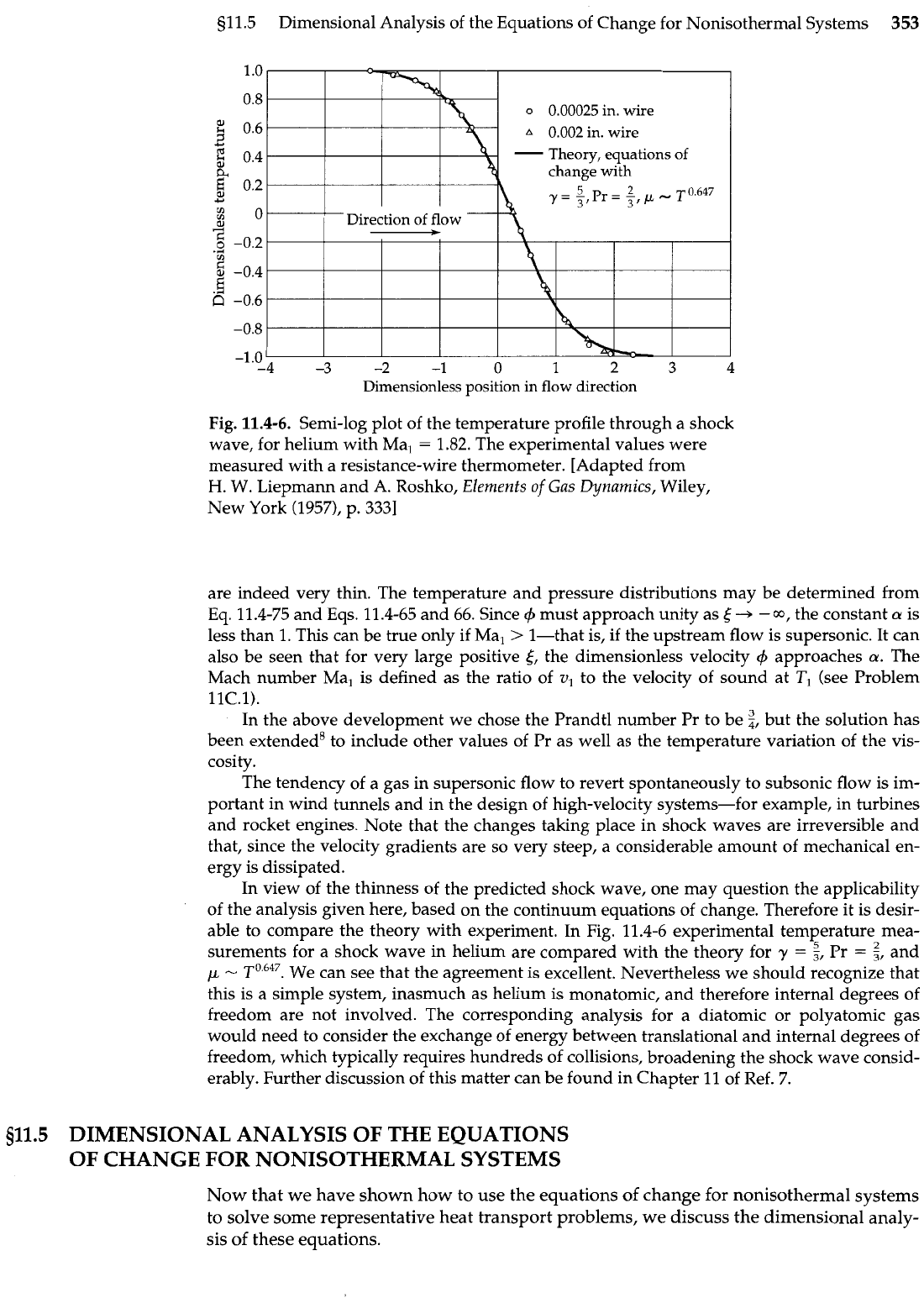
Fig.
11.4-6.
Semi-log plot of the temperature profile through a shock
wave, for helium with Ma,
=
1.82.
The experimental values were
measured with a resistance-wire thermometer. [Adapted from
H.
W. Liepmann and A. Roshko, Elements
of
Gas Dynamics, Wiley,
New York (1957), p.
3331
are indeed very thin. The temperature and pressure distributions may be determined from
Eq. 11.4-75 and Eqs. 11.4-65 and 66. Since
4
must approach unity as
6
-+
-a,
the constant
a
is
less than 1. This can be true only if Ma,
>
1-that is, if the upstream flow is supersonic. It can
also be seen that for very large positive 6, the dimensionless velocity
4
approaches
a.
The
Mach number Ma, is defined as the ratio of
v,
to the velocity of sound at
TI
(see Problem
11C.1).
In the above development we chose the Prandtl number Pr to be
z,
but the solution has
been extended8 to include other values of Pr as well as the temperature variation of the vis-
cosity.
The tendency of a gas in supersonic flow to revert spontaneously to subsonic flow is im-
portant in wind tunnels and in the design of high-velocity systems-for example, in turbines
and rocket engines. Note that the changes taking place in shock waves are irreversible and
that, since the velocity gradients are so very steep, a considerable amount of mechanical en-
ergy is dissipated.
In view of the thinness of the predicted shock wave, one may question the applicability
of the analysis given here, based on the continuum equations of change. Therefore it is desir-
able to compare the theory with experiment. In Fig. 11.4-6 experimental temperature mea-
surements for a shock wave in helium are compared with the theory for
y
=
z,
Pr
=
z,
and
p
-
We can see that the agreement is excellent. Nevertheless we should recognize that
this is a simple system, inasmuch as helium is monatomic, and therefore internal degrees of
freedom are not involved. The corresponding analysis for a diatomic or polyatomic gas
would need to consider the exchange of energy between translational and internal degrees of
freedom, which typically requires hundreds of collisions, broadening the shock wave consid-
erably. Further discussion of this matter can be found in Chapter
11
of Ref. 7.
511.5
DIMENSIONAL ANALYSIS OF THE EQUATIONS
OF CHANGE FOR NONISOTHERMAL SYSTEMS
Now that we have shown how to use the equations
of
change for nonisothermal systems
to solve some representative heat transport problems, we discuss the dimensional analy-
sis of these equations.
354
Chapter 11 The Equations of Change for Nonisothermal Systems
Just as the dimensional analysis discussion in 53.7 provided an introduction for the
discussion of friction factors in Chapter
6,
the material in this section provides the back-
ground needed for the discussion of heat transfer coefficient correlations in Chapter 14.
As in Chapter 3, we write the equations of change and boundary conditions in dimen-
sionless form. In this way we find some dimensionless parameters that can be used
to
characterize nonisothermal flow systems.
We shall see, however, that the analysis of nonisothermal systems leads us to
a
larger number of dimensionless groups than we had in Chapter 3. As a result, greater re-
liance has to be placed on judicious simplifications of the equations of change and on
carefully chosen physical models. Examples of the latter are the Boussinesq equation
of
motion for free convection (511.3) and the laminar boundary layer equations (512.4).
As in 53.7, for the sake of simplicity we restrict ourselves to a fluid with constant
p,
k,
and
tp.
The density is taken to be
p
=
p
-
?F(T
-
n
in the
pg
term in the equation
of
motion, and
p
=
p
everywhere else (the "Boussinesq approximation"). The equations
of
change then become with
p
+
sgh
expressed as
9,
Continuity:
(V
v)
=
0 (1 1.5-1)
Motion:
Energy:
We now introduce quantities made dimensionless with the characteristic quantities (sub-
script 0 or 1) as follows:
Here lo,
v,,
and
Po
are the reference quantities introduced in s3.7, and
T,
and
TI
are
temperatures appearing in the boundary conditions. In Eq. 11.5-2 the value
7.
is the
temperature around which the density
p
was expanded.
In terms of these dimensionless variables, the equations of change in Eqs. 11.5-1 to
3
take the forms
Energy:
The characteristic velocity can be chosen in several ways, and the consequences of
the
choices are summarized in Table 11.5-1. The dimensionless groups appearing in
Eqs.
11.5-8 and
9,
along with some combinations of these groups, are summarized in Table
11.5-2. Further dimensionless groups may arise in the boundary conditions or in the
equation of state. The Froude and Weber numbers have already been introduced in
93.7,
and the Mach number in Ex. 11.4-7.
We already saw in Chapter 10 how several dimensionless groups appeared in the
solution of nonisothermal problems. Here we have seen that the same groupings appear
naturally when the equations of change are made dimensionless. These dimensionless
groups are used widely in correlations of heat transfer coefficients.
s11.5
Dimensional Analysis of the Equations of Change for Nonisothermal Systems
355
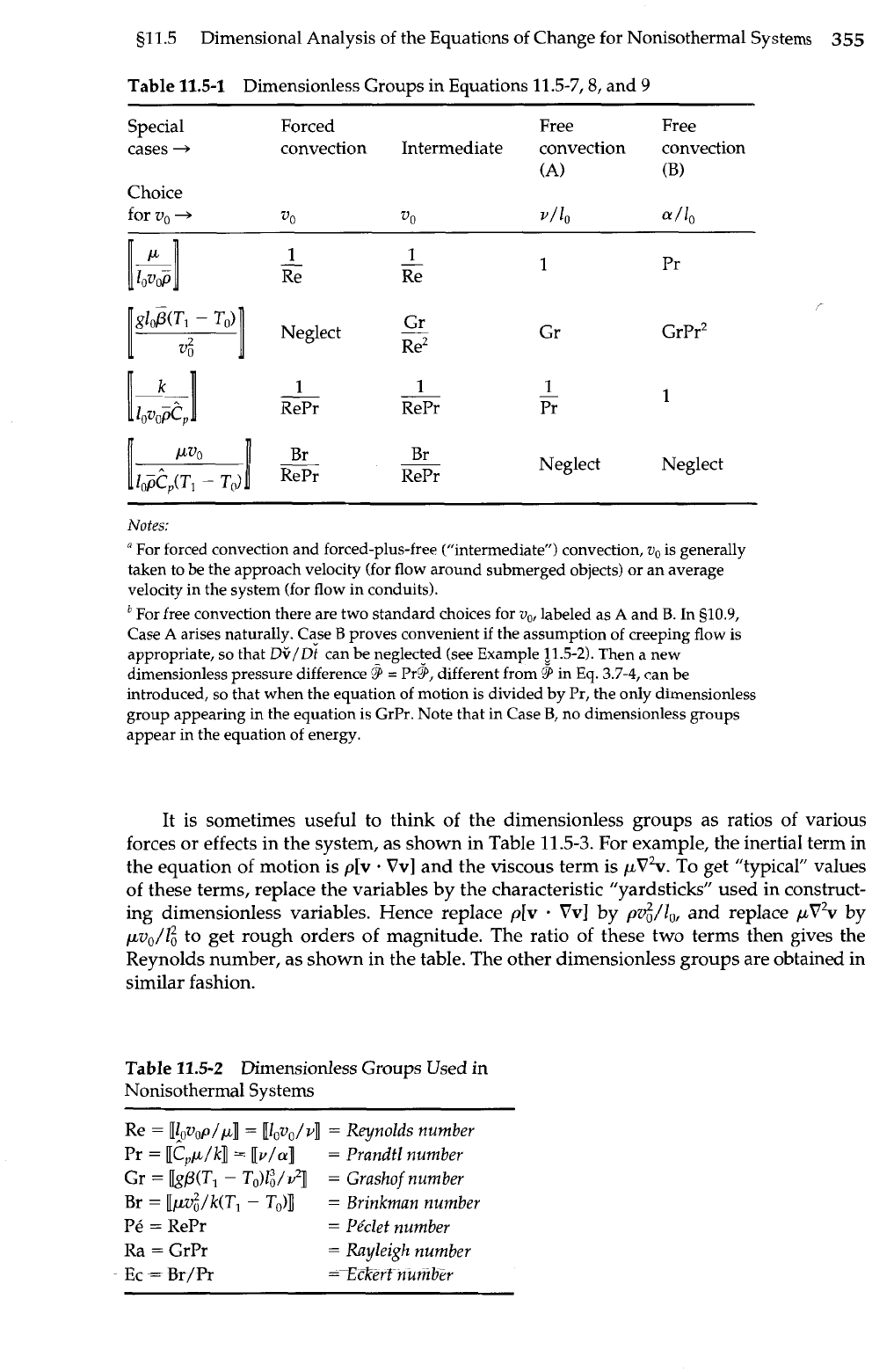
Table
11.5-1
Dimensionless Groups in Equations 11.5-7,8, and
9
Special Forced Free Free
cases
+
convection Intermediate convection convection
(A)
(B)
Choice
for
vo
-+
Vo
Vo
v/10
a
/lo
1
1
-
-
RePr RePr
Neglect Neglect
Notes:
"
For forced convection and forced-plus-free ("intermediate") convection,
v,
is generally
taken to be the approach velocity (for flow around submerged objects) or an average
velocity in the system (for flow in conduits).
For free convection there are two standard choices for
v,,
labeled as
A
and
B.
In
g10.9,
Case
A
arises naturally. Case
B
proves convenient if the assumption of creeping flow is
appropriate, so that
D+/D~
can be neglected (see Example
11.5-2).
Then a new
dimensionless pressure difference
9
=
Pry, different from
9
in Eq.
3.7-4,
can be
introduced, so that when the equation of motion is divided by Pr, the only dimensionless
group appearing in the equation is GrPr. Note that in Case
B,
no dimensionless groups
appear in the equation of energy.
It is sometimes useful to think of the dimensionless groups as ratios of various
forces or effects in the system, as shown in Table
11.5-3.
For example, the inertia1 term in
the equation
of
motion is
p[v
.
Vvl
and the viscous term is To get "typical" values
of these terms, replace the variables by the characteristic "yardsticks" used in construct-
ing
dimensionless variables. Hence replace p[v
-
Vv]
by p@lo, and replace
/AV%
by
,!~v~/li to get rough orders of magnitude. The ratio of these two terms then gives the
Reynolds number, as shown in the table. The other dimensionless groups are obtained in
similar fashion.
Table
11.5-2
Dimensionless Groups Used in
Nonisothermal Systems
Re
=
[lov,p/p]l
=
[lov,/v]l
=
Reynolds number
Pr
=
[epp/k]
=
[v/a]
=
Prandtl number
Gr
=
bp(T,
-
T,)I;/S?]
=
Grashof number
Br
=
[[pv~/k(~l
-
To)]
=
Brinkman number
Pe
=
RePr
=
Pkclet number
Ra
=
GrPr
=
Rayleigh number
Ec
=
&-/R
=Z&erf number
356
Chapter
11
The Equations of Change for Nonisothermal Systems
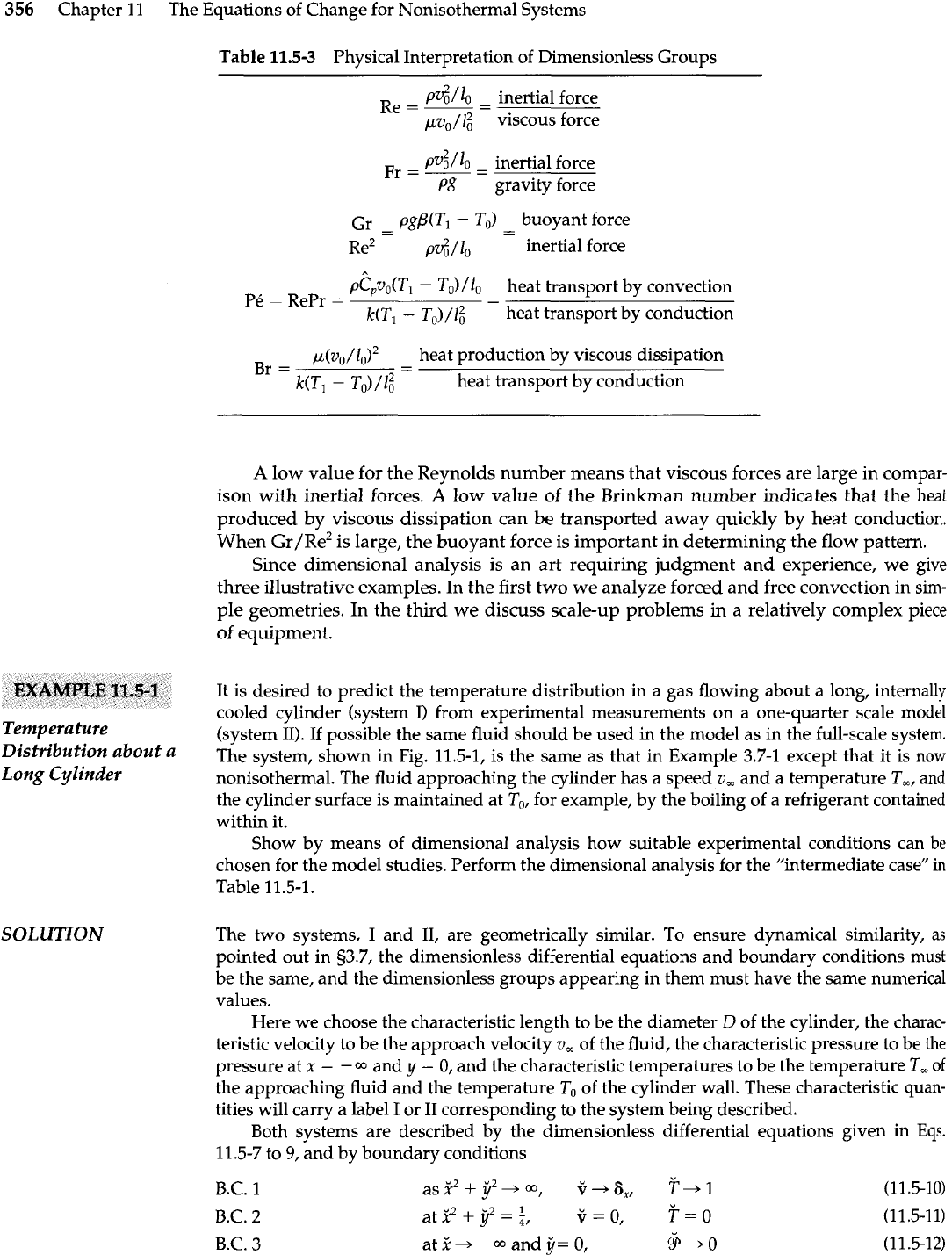
Table
11.5-3
Physical Interpretation of Dimensionless Groups
~v?j/lo
-
inertial force
Re=--
v0/ viscous force
pv?j/Io
-
inertial force
Fr=--
P8 gravity force
Gr
-
pg/3(Tl
-
To)
-
buoyant force
--
-
Re2
PV?~
/
10
inertial force
~C,V&T~
-
TJ
/
10
heat transport by convection
Pk
=
RePr
=
-
--
(
-
)
heat transport by conduction
p(vo/lo)2
heat production by viscous dissipation
Br
=
- -
kU-1
-
To)/G
heat transport by conduction
A
low value for the Reynolds number means that viscous forces are large in compar-
ison with inertial forces.
A
low value of the Brinkrnan number indicates that the heat
produced by viscous dissipation can
be
transported away quickly by heat conduction.
When ~r/~e* is large, the buoyant force is important in determining the flow pattern.
Since dimensional analysis is an art requiring judgment and experience, we give
three illustrative examples. In the first two we analyze forced and free convection in sim-
ple geometries. In the third we discuss scale-up problems in a relatively complex piece
of equipment.
EXAMPLE
125-1
Temperature
Distribution about
a
Long
Cylinder
It is desired to predict the temperature distribution in a gas flowing about a long, internally
cooled cylinder (system
I)
from experimental measurements on a one-quarter scale model
(system 11). If possible the same fluid should be used in the model as in the full-scale system.
The system, shown in Fig. 11.5-1, is the same as that in Example 3.7-1 except that it is now
nonisothermal. The fluid approaching the cylinder has a speed
v,
and a temperature T,, and
the cylinder surface is maintained at To, for example, by the boiling of a refrigerant contained
within it.
Show by means of dimensional analysis how suitable experimental conditions can be
chosen for the model studies. Perform the dimensional analysis for the "intermediate case" in
Table 11.5-1.
SOLUTION
The two systems, I and 11, are geometrically similar. To ensure dynamical similarity, as
pointed out in 53.7, the dimensionless differential equations and boundary conditions must
be the same, and the dimensionless groups appearing in them must have the same numerical
values.
Here we choose the characteristic length to be the diameter
D
of the cylinder, the charac-
teristic velocity to be the approach velocity
v,
of the fluid, the characteristic pressure to be the
pressure at
x
=
-
03
and
y
=
0, and the characteristic temperatures to be the temperature T,
of
the approaching fluid and the temperature To of the cylinder wall. These characteristic quan-
tities will carry a label
I
or I1 corresponding to the system being described.
Both systems are described by the dimensionless differential equations given in
Eqs.
11.5-7 to
9,
and by boundary conditions
B.C. 1
B.C.
2
B.C.
3
