Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

s9.3
Theory of Thermal Conductivity of Gases at Low Density
277
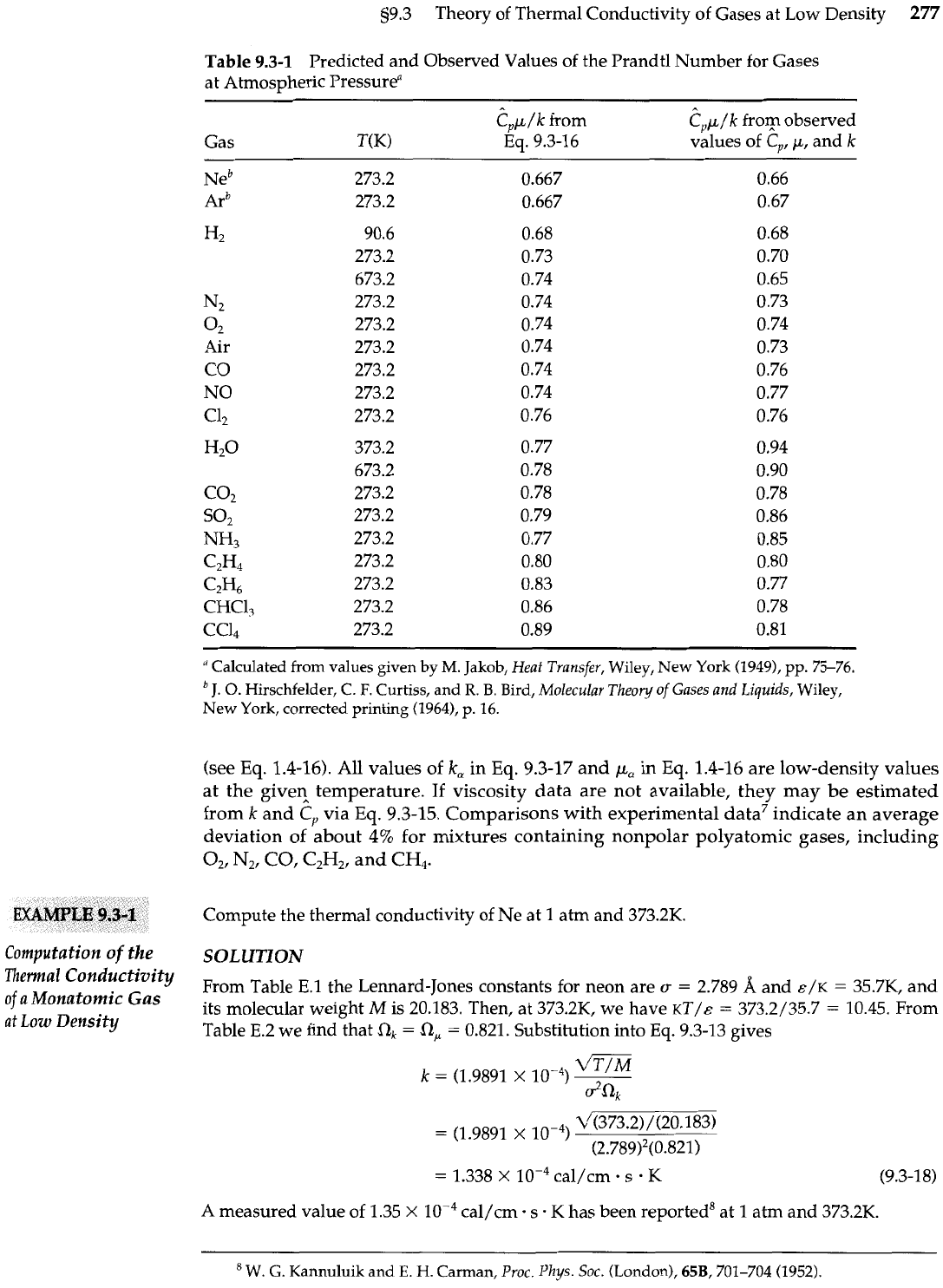
Table
9.3-1
Predicted and Observed Values of the Prandtl Number for Gases
at Atmospheric Pressurea
-
--
&~/k
from
ePr
/
k
from observed
Gas T(K)
Eq.
9.3-16 values of
C,,
p, and
k
N2
02
Air
CO
NO
c4
"
Calculated from values given
by
M.
Jakob,
Heaf Transfer,
Wiley,
New York (19491,
pp.
75-76.
J.
0.
Hirschfelder,
C. F.
Curtis, and
R.
B.
Bird,
Molecular Theory
of
Gases and Liquids,
Wiley,
New York, corrected printing (1964),
p.
16.
(see
Eq.
1.4-16).
All
values of
k,
in
Eq.
9.3-17 and
p,
in
Eq.
1.4-16 are low-density values
at the give; temperature.
If
viscosity data are not available, they may be estimated
from
k
and
C,
via
Eq.
9.3-15. Comparisons with experimental data7 indicate an average
deviation of about
4%
for mixtures containing nonpolar polyatomic gases, including
02,
N2,
CO,
C2H2, and CH,.
Compute the thermal conductivity of Ne at
1
atm and 373.2K.
Computation
of
the
SOLUTION
lhenal
Conductivity
From Table E.l the Lennard-Jones constants for neon are
u
=
2.789
A
and
E/K
=
35.7K, and
'fa
Monatomic
Gas
its molecular weight
M
is 20.183. Then, at 373.2K,
we
have KT/E
=
373.2/35.7
=
10.45. From
at
Low
Density
Table E.2 we find that
flk
=
fl,
=
0.821. Substitution into Eq. 9.3-13 gives
A
measured value of 1.35
X
cal/cm. s
.
K
has been reported8 at 1 atm and 373.2K.
W.
G.
Kannuluik and
E.
H.
Carman,
Proc. Phys. Soc.
(London),
65B,
701-704 (1952).
278
Chapter 9 Thermal Conductivity and the Mechanisms of Energy Transport
Estimation of the
Thermal Conductivity
of a Polyatomic Gas
at Low Density
EXAMPLE
9.3-3
Prediction of the
Thermal
Conductivity
of
a Gas Mixture at
Low Density
Estimate the thermal conductivity of molecular oxygen at 300K and low pressure.
SOLUTION
The molecular weight of
O2
is 32.0000; its molar heat capacity
tp
at 300°K and low pressure
is
7.019 cal/g-mole
.
K.
From Table E.l we find the Lennard-Jones parameters for molecular
oxygen to be
a
=
3.433
A
and
E/K
=
113K. At 300K, then,
KT/E
=
300/113
=
2.655. From
Table E.2, we find
R,
=
1.074. The viscosity, from Eq. 1.4-14, is
Then, from Eq. 9.3-15, the Eucken approximation to the thermal conductivity is
This compares favorably with the experimental value of 0.02657 W/m
K
in Table 9.1-2.
Predict the thermal conductivity of the following gas mixture at 1 atm and 293K from the
given data on the pure components at the same pressure and temperature:
-
-
Mole Molecular
fraction weight
x
lo7
k,
x
lo7
Species
a
X,
M,
(g/cm. s)
(cal/cm
-
s
.
K)
SOLUTION
Use Eq. 9.3-17. We note that the for this gas mixture at these conditions have already
been
computed in the viscosity calculation in Example 1.4-2. In that example we evaluated the fol-
lowing summations, which also appear in
Eq.
9.3-17:
Substitution in
Eq.
9.3-17 gives
No
data are available for comparison at these conditions.

59.4
Theory of Thermal Conductivity of Liquids
279
59.4
THEORY OF THERMAL CONDUCTIVITY OF LIQUIDS
A
very detailed kinetic theory for the thermal conductivity of monatomic liquids was
developed a half-century ago,' but it has not yet been possible to implement it for prac-
tical calculations. As a result we have to use rough theories or empirical estimation
methods.'
We choose to discuss here Bridgman's simple theory3 of energy transport in pure
liquids. He assumed that the molecules are arranged in a cubic lattice, with a center-
to-center spacing given by in which
?/N
is the volume per molecule. He
further assumed energy to be transferred from one lattice plane to the next at the
sonic velocity
v,
for the given fluid. The development is based on a reinterpretation of
Eq. 9.3-11 of the rigid-sphere gas theory:
The heat capacity at constant volume of a monatomic liquid is about the scme as for a
solid at high temperature, which is given by the Dulong and Petit formula4
Cv
=
3(~/m).
The mean molecular speed in the
y
direction,
m,
is replaced by the sonic velocity
us.
The
distance a that the energy travels between two successive collisions is taken to be the lat-
tice spacing
(?/I?)'13.
Making these substitutions in Eq. 9.4-1 gives
which is Bridgrnan's equation. Experimental data show good agreement with Eq. 9.4-2,
even for polyatomic liquids, but the numerical coefficient is somewhat too high. Better
agreement is obtained if the coefficient is changed to
2.80:
This equation is limited to densities well above the critical density, because of the tacit
assumption that each molecule oscillates in a "cage" formed by its nearest neighbors.
The success of this equation for polyatomic fluids seems to imply that the energy trans-
fer
in
collisions of polyatomic molecules is incomplete, since the heat capacity used here,
kv
=
3(~/rn), is less than the heat capacities of polyatomic liquids.
The
velocity of low-frequency sound is given (see Problem 11C.1) by
The quantity (dp/dp), may be obtained from isothermal compressibility measurements
or from an equation of state, and (Cp/Cv) is very nearly unity for liquids, except near the
critical point.
'
J.
H.
Irving and
J.
G. Kirkwood,
I.
Chem. Phys.,
18,817-829
(1950).
This theory has been extended
to polymeric liquids by
C.
F.
Curtiss and R. B. Bird,
J.
Chem. Phys.,
107,5254-5267 (1997).
R.
C.
Reid,
J.
M. Prausnitz, and
B.
E.
Poling,
The Properties of Gases and
Liquids,
McGraw-Hill,
New York
(1987);
L.
Riedel,
Chemie-1ng.-Techn.,
27,209-213
(1955).
".
W.
Bridgman,
Proc. Am. Acad. Arts and Sci.,
59,141-169 (1923).
Bridgman's equation is often
misquoted, because he gave it in terms of a little-known gas constant equal to
SK.
This empirical equation has been justified, and extended, by
A.
Einstein
[Ann. Phys.
[41,22,
180-190 (1907)l
and
P.
Debye
[Ann.
Phys.,
[4139,789-839 (1912)l.
Equation
9.4-3
is in approximate agreement with a formula derived by
R.
E.
Powell,
W.
E.
Roseveare, and
H.
Eyring,
Ind. Eng. Chem.,
33,430-435
(1941).

280
Chapter 9
Thermal Conductivity and the Mechanisms of Energy Transport
EXAMPLE
9.4-1
Prediction
of
the
The density of liquid CCI, at
20°C
and 1 atm is 1.595 g/cm3, and its isothermal compressibility
(l/p)(ap/a~)~ is 90.7
X
atm-'. What is its thermal conductivity?
Thermal Conductivity
SOLUTION
of a Liquid
First compute
=
7.00
X
lo9
cm2/s2 (using Appendix
F)
(9.4-5)
Assuming that
CJC,
=
1.0,
we
get from
Eq.
9.4-4
The molar volume is
=
M/p
=
153.84/1.595
=
96.5 cm3/g-mole. Substitution of these val-
ues in Eq. 9.4-3 gives
The experimental value as interpolated from Table 9.1-3 is 0.101 W/m
K.
s9.5
THERMAL CONDUCTIVITY OF SOLIDS
Thermal conductivities of solids have to be measured experimentally, since they depend
on many factors that are difficult to measure or predict.' In crystalline materials, the
phase and crystallite size are important; in amorphous solids the degree of molecular
orientation has a considerable effect. In porous solids, the thermal conductivity is
strongly dependent on the void fraction, the pore size, and the fluid contained in the
pores.
A
detailed discussion of thermal conductivity of solids has been given by Jakob.'
In general, metals are better heat conductors than nonmetals, and crystalline materi-
als conduct heat more readily than amorphous materials.
Dry
porous solids are very
poor heat conductors and are therefore excellent for thermal insulation. The conductivi-
ties of most pure metals decrease with increasing temperature, whereas the conductivi-
ties of nonmetals increase; alloys show intermediate behavior. Perhaps the most useful
of the rules of thumb is that thermal and electrical conductivity go hand in hand.
For pure metals, as opposed to alloys, the thermal conductivity
k
and the electrical
conductivity
k,
are related approximately3 as follows:
--
-
L
=
constant
kJ
This is the
Wiedemann-Franz-Lorenz equation;
this equation can also be explained theoret-
ically (see Problem
9A.6).
The "Lorenz number"
L
is about
22
to
29
X
lop9
volt2/K2 for
A.
Goldsmith,
T.
E.
Waterman, and H. J. Hirschhorn, eds.,
Handbook of Thermophysical Properfies of
Solids,
Macmillan, New York (1961).
M.
Jakob,
Heat Transfer,
Vol. 1, Wiley, New York (1949), Chapter
6.
See also
W.
H.
Rohsenow,
J.
P.
Hartnett, and Y.
I.
Cho, eds., Handbook of
Heat
Transfer,
McGraw-Hill, New York (1998).
%.
Wiedemann and
R.
Franz,
Ann. Phys.
u.
Chernie,
89,497-531
(1853);
L.
Lorenz,
Poggendorff's
Annalen,
147,429-452
(1872).

s9.6
Effective Thermal Conductivity of Composite Solids
281
pure metals at 0°C and changes but little with temperatures above O°C, increases of
10-20% per 1000°C being typical. At very low temperatures (-269.4"C for mercury) met-
als become superconductors of electricity but not of heat, and
L
thus varies strongly with
temperature near the superconducting region. Equation 9.5-1 is of limited use for alloys,
since
L
varies strongly with composition and, in some cases, with temperature.
The success of
Eq.
9.5-1 for pure metals is due to the fact that free electrons are the
major heat carriers in pure metals. The equation is not suitable for nonmetals, in which
the concentration of free electrons is so low that energy transport by molecular motion
predominates.
59.6
EFFECTIVE THERMAL CONDUCTIVITY
OF
COMPOSITE SOLIDS
Up to this point we have discussed homogeneous materials. Now we turn our attention
briefly to the thermal conductivity of two-phase solids--one solid phase dispersed in a
second solid phase, or solids containing pores, such as granular materials, sintered met-
als, and plastic foams. A complete description of the heat transport through such materi-
als is clearly extremely complicated. However, for steady conduction these materials can
be regarded as homogeneous materials with an
effective thermal conductivity
keff,
and the
temperature and heat flux components are reinterpreted as the analogous quantities av-
eraged over a volume that is large with respect to the scale of the heterogeneity but small
with respect to the overall dimensions of the heat conduction system.
The first major contribution to the estimation of the conductivity of heterogeneous
solids was by Maxwell.' He considered a material made of spheres of thermal conductiv-
ity
k,
embedded in a continuous solid phase with thermal conductivity
ko.
The volume
fraction
4
of embedded spheres is taken to be sufficiently small that the spheres do not
"interact" thermally; that is, one needs to consider only the thermal conduction in a large
medium containing only one embedded sphere. Then by means of a surprisingly simple
derivation, Maxwell showed that for
small volume fraction
4
(see Problems llB.8 and llC.5).
For
large volume fraction
4,
Rayleigh2 showed that, if the spheres are located at the
in-
tersections of a cubic lattice, the thermal conductivity of the composite is given by
Comparison of this result with
Eq.
9.6-1 shows that the interaction between the spheres
is small, even at
4
=
in,
the maximum possible value of
4
for the cubic lattice arrange-
ment. Therefore the simpler result of Maxwell is often used, and the effects of nonuni-
form sphere distribution are usually neglected.
Maxwell's derivation was for electrical conductivity, but
the
same arguments apply for thermal
conductivity. See J.
C.
Maxwell,
A
Treatise on Electricity and Magnetism,
Oxford University Press, 3rd
edition (1891, reprinted 1998), Vol. 1,
s314;
H.
S. Carslaw and
J.
C.
Jaeger,
Conduction
of
Heat in Solids,
Clarendon Press, Oxford, 2nd edition (1959),
p.
428.
J.
W.
Strutt (Lord Rayleigh),
Phil.
Mag.
(5),
34,431-502 (1892).
282
Chapter
9
Thermal Conductivity and the Mechanisms of Energy Transport
For nonspherical inclusions, however,
Eq.
9.6-1 does require modification. Thus for
square arrays of long cylinders parallel to the
z
axis, ~aylei~h~ showed that the
zz
com-
ponent of the thermal conductivity tensor
K
is
and the other two components are
Keff,
xx
Keff,
yy
-
-
1
+
24
(9.6-4)
ko
ko
kl
-
ko
(0.305844~
+
0.0133634~
+
. .
-1
That is,
the composite solid containing aligned embedded cylinders is anisotropic. The effective
thermal conductivity tensor has been computed up to
0(+')
for a medium containing
spheroidal
inclusion^.^
For complex nonspherical inclusions, often encountered in practice, no exact treatment
is possible, but some approximate relations are a~ailable.~,~,~ For simple unconsolidated
granular beds the following expression has proven successful:
in
which
The
gk
are "shape factors" for the granules of the medium: and they must satisfy g,
t
g2
+
g3
=
1.
For spheres g1
=
g2
=
g3
=
&
and
Eq.
9.6-5 reduces to
Eq.
9.6-1. For unconsol-
idated soils? gl
=
g2
=
d
and g3
=
2.
The structure of consolidated porous beds-for ex-
ample, sandstones-is considerably more complex.
Some success is claimed for
predicting the effective conductivity of such s~bstances;l.~,~ but the generality of the
methods is not yet known.
For solids containing gas
pocket^,^
thermal radiation (see Chapter 16) may be impor-
tant. The special case
of
parallel planar fissures perpendicular to the direction of heat
conduction is particularly important for high-temperature insulation. For such systems
it
may be shown that
where
a
is the Stefan-Boltzmann constant,
k1
is the thermal conductivity of the gas, and
L
is the total thickness of the material in the direction of the heat conduction.
A
modifica-
tion of this equation for fissures of other shapes and orientations is a~ailable.~
S.-Y.
Lu
and
S.
Kim,
AIChE lournal,
36,927-938 (1990).
".
I.
Odelevskii,
J.
Tech. Phys.
(USSR), 24,667
and
697 (1954);
F.
Euler,
J.
Appl. Phys.,
28,1342-1346
(1957).
D.
A.
de Vries,
Mededelingen van de Landbouwhogeschool te Wageningen,
(1952);
see also
Ref.
6
and
D. A.
de Vries, Chapter
7
in
Physics
of
Plant Environment,
W.
R.
van Wijk, ed., Wiley,
New
York
(1963).
W. Woodside and
J.
H.
Messmer,
J.
Appl. Phys.,
32,1688-1699,1699-1706 (1961).
A.
L.
Loeb,
J.
Amer. Ceramic Soc.,
37,96-99 (1954).
Sh.
N.
Plyat,
Soviet Physics
JETP,
2,2588-2589 (1957).
M. Jakob,
Heat Transfer,
Wiley,
New
York
(1959),
Vol.
1,§6.5.
59.7
Convective Transport of Energy
283
For
gas-filled granular
beds6r9
a different type of complication arises. Since the thermal
conductivities of gases are much lower than those of solids, most of the gas-phase heat
conduction is concentrated near the points of contact of adjacent solid particles. As a re-
sult, the distances over which the heat is conducted through the gas may approach the
mean free path of the gas molecules. When this is true, the conditions for the develop-
ments of 59.3 are violated, and the thermal conductivity of the gas decreases. Very effec-
tive insulators can thus be prepared from partially evacuated beds of fine powders.
Cylindrical ducts filled with granular materials through which a fluid
is
flowing
(in
the
z
di-
rection) are of considerable importance in separation processes and chemical reactors. In
such systems the effective thermal conductivities in the radial and axial directions are
quite different and are designated1' by
K~~~,~~
and
K,,,,,.
Conduction, convection, and radia-
tion all contribute to the flow of heat through the porous medium." For highly turbulent
flow, the energy is transported primarily
by
the tortuous flow of the fluid in the inter-
stices of the granular material; this gives rise to a highly anisotropic thermal conductivity.
For a bed of uniform spheres, the radial and axial components are approximately
in which vo is the "superficial velocity" defined in 54.3 and 56.4, and
D,
is the diameter of
the spherical particles. These simplified relations hold for Re
=
D,vop/p greater than
200.
The behavior at lower Reynolds numbers is discussed in several references.12 Also,
the behavior of the effective thermal conductivity tensor as a function of the Pkclet num-
ber has been studied in considerable detail.13
59.7
CONVECTIVE
TRANSPORT OF
ENERGY
In 59.1 we gave Fourier's law of heat conduction, which accounts for the energy trans-
ported through a medium by virtue of the molecular motions.
Energy may also be transported by the bulk motion of the fluid. In Fig. 9.7-1 we
show three mutually perpendicular elements of area
dS
at the point
P,
where the fluid
Fig.
9.7-1.
Three mutually perpendicular surface elements of area dS across which energy is
being transported by convection by the fluid moving with the velocity
v.
The
volume rate of
flow across the faceperpendicular to the x-axis is v,dS, and the rate of flow of energy across
dS
is then
(ipv2
+
pU)v,dS. Similar expressions can be written for the surface elements per-
pendicular to the
y-
and z-axes.
In
See
Eq.
9.1-7
for the modification of Fourier's law for anisotropic materials. The subscripts
rr
and
zz
emphasize that these quantities are components of a second-order symmetrical tensor.
"W. B. Argo and
J.
M.
Smith,
Chem. Engr. Progress,
49,443-451 (1953).
l2
J.
Beek,
Adv. Chem. Engr.,
3,203-271 (1962);
H.
Kramers and
K.
R.
Westerterp,
Elements
of
Chemical
Reacfor Design and Operation,
Academic Press, New York
(1963), gIII.9;
0.
Levenspiel and
K.
B. Bischoff,
Adv. Chem. Engr.,
4,95-198 (1963).
l3
D.
L.
Koch
and
J.
F.
Brady,
J.
Fluid Mech.,
154,399427 (1985).

284
Chapter
9
Thermal Conductivity and the Mechanisms of Energy Transport
velocity is v. The volume rate of flow across the surface element dS perpendicular to the
x-axis is v,dS. The rate at which energy is being swept across the same surface element is
then
in which $pv2
=
$p(v:
+
4
+
vi)
is the kinetic energy per unit volume, and pir is the inter-
nal energy per unit volume.
The definition of the internal energy in a nonequilibrium situation requires some
care. From the continuum point of view, the internal energy at position r and time t is as-
sumed to be the same function of the local, instantaneous density and temperature that
one would have at equilibrium. From the molecular point of view, the internal energy
consists of the sum of the kinetic energies of all the constituent atoms (relative to the
flow velocity v), the intramolecular potential energies, and the intermolecular energies,
within a small region about the point
r
at time t.
Recall that, in the discussion of molecular collisions in 50.3, we found it convenient
to regard the energy of a colliding pair of molecules to be the sum of the kinetic energies
referred to the center of mass of the molecule plus the intramolecular potential energy
of
the molecule. Here also we split the energy of the fluid (regarded as a continuum) into
kinetic energy associated with the bulk fluid motion and the internal energy associated
with the kinetic energy of the molecules with respect to the flow velocity and the intra-
and intermolecular potential energies.
We can write expressions similar to Eq. 9.7-1 for the rate at which energy is being
swept through the surface elements perpendicular to the
y-
and z-axes. If we now multi-
ply each of the three expressions by the corresponding unit vector and add, we then get,
after division by dS,
and this quantity is called the convective energy flux vector. To get the convective energy
flux across a_ unit surface whose normal unit vector is
n,
we form the dot product
(n
.
($v2
+
pU)v). It is understood that this is the flux from the negative side of the sur-
face to the positive side. Compare this with the convective momentum flux in Fig. 1.7-2.
g9.8
WORK ASSOCIATED
WITH
MOLECULAR MOTIONS
Presently we will be concerned with applying the law of conservation of energy to
"shells" (as in the shell balances in Chapter 10) or to small elements of volume fixed
in
space (to develop the equation of change for energy in §11.1). The law of conservation
of
energy for an open flow system is an extension of the first law of classical thermodynam-
ics (for a closed system at rest). In the latter we state that the change in internal energy is
equal to the amount of heat added to the system plus the amount of work done on the
system. For flow systems we shall need to account for the heat added to the system (by
molecular motions and by bulk fluid motion) and also for the work done on the system
by the molecular motions. Therefore it is appropriate that we develop here the expres-
sion for the rate of work done by the molecular motions.
First we recall that, when a force F acts on a body and causes it to move through a
distance dr, the work done is dW
=
(F dr). Then the rate of doing work is dW/dt
=
(F
.
drldt)
=
(F
v)-that is, the dot product of the force times the velocity. We now
apply this formula to the three perpendicular planes at a point
P
in space shown
in
Fig.
9.8-1.
First we consider the surface element perpendicular to the x-axis. The fluid on the
minus side of the surface exerts a force
IT#
on the fluid that is on the plus side (see

g9.8
Work Associated with Molecular Motions
285
Fig.
9.8-1.
Three mutually perpendicular surface elements of area dS at point
P
along with
the stress vectors
m,, my,
n,
acting on these surfaces. In the first figure, the rate at which
work is done
by
the
fluid
on the minus side of dS on the fluid on the plus side of
dS
is then
(a,.
v)dS
=
[m
v],dS. Similar expressions hold for the surface elements perpendicular to
the other two coordinate axes.
Table 1.2-1). Since the fluid is moving with a velocity v, the rate at which work is done
by the minus fluid on the plus fluid is
(n,
v)dS. Similar expressions may be written for
the work done across the other two surface elements. When written out in component
form, these rate of work expressions, per unit area, become
When these scalar components are multiplied by the unit vectors and added, we get the
"rate of doing work vector per unit area," and we can call this, for short, the work flux:
Furthermore, the rate of doing work across a unit area of surface with orientation given
by the unit vector n is (n
.
[n
-
v]).
Equations 9.8-1 to 9.8-4 are easily written for cylindrical coordinates by replacing
x,
y,
z
by
r,
8,
z
and, for spherical coordinates by replacing x,
y,
z
by
r,
6,
4.
We now define, for later use, the combined energy flux vector
e
as follows:
The
e
vector is the sum of (a) the convective energy flux,
(b)
the rate of doing work (per
unit area) by molecular mechanisms, and (c) the rate of transporting heat (per unit area)
by molecular mechanisms. All the terms in
Eq.
9.8-5 have the same sign convention, so
that
ex
is the energy transport
in
the positive
x
direction per unit area per unit time.
The total molecular stress tensor
.rr
can now be split into two parts:
n
=
p6
+
T
so that
[n
.
v]
=
pv
+
[T
v]. The term
py
can then be combined with !he internal
energy term to give an enthalpy term pUv
+
pv
=
p(~
+
(p/p))v
=
p(U
+
pi3v
=
&v, so that
e
=
($pv2
+
pkv
+
[T
.
v]
+
q
(9.8-6)
We shall usually use the
e
vector in this form. For a surface element dS of orientation n,
the quantity (n
.
e)
gives the convective energy flux, the heat flux, and the work flux
across the surface element dS from the negative side to the positive side of dS.

286
Chapter 9
Thermal Conductivity and the Mechanisms of Energy Transport
Table
9.8-1
Summary of Notation for Energy Fluxes
Symbol Meaning Reference
(ipv2
+
pinv convective energy flux vector Eq. 9.7-2
molecular heat flux vector Eq. 9.1-6
molecular work flux vector Eq. 9.8-4
e
=
q
+
[P
.
VI
+
(ipv2
+
pkv
combined energy flux vector
Eq. 9.8-5,6
=
q
+
[7
'
"1
+
(ipv2
+
pkv
In Table 9.8-1 we summarize the notation for the various energy flux vectors intro-
duced in this section. All of them have the same sign convention.
To evaluate the enthalpy in
Eq.
9.8-6,
we make use of the standard equilibrium ther-
modynamics formula
A
($),
(z;)~
A
dH=
-
dT+
-
dp=C,dT+ V-T
-
[A
(:P))p
When this is integrated from some reference state pol To to the state p, TI we then get1
in which H" is the enthalpy per unit mass at the reference state. The integral over
p
is
zero for a? ideal gas and (l/p)(p
-
pO)
for fluids of constant density. The integral over
T
becomes C,(T
-
To) if the heat capacity can be regarded as constant over the relevant
temperature range. It is assumed that
Eq.
9.8-7 is valid in nonequilibrium systems,
where p and Tare the
local
values of the pressure and temperature.
QUESTIONS FOR DISCUSSION
1.
Define and give the dimensions of thermal conductivity
k,
thermal diffusivity
a,
heat capacity
C,,
heat flux q, and combined energy flux
e.
For the dimensions use
m
=
mass,
I
=
length,
T
=
temperature, and
t
=
time.
2.
Compare the orders of magnitude of the thermal conductivities of gases, liquids, and solids.
3.
In what way are Newton's law of viscosity and Fourier's law of heat conduction similar? Dis-
similar?
4.
Are gas viscosities and thermal conductivities related? If so, how?
5.
Compare the temperature dependence of the thermal conductivities of gases, liquids, and
solids.
6.
Compare the orders of magnitudes of Prandtl numbers for gases and liquids.
7.
Are the thermal conductivities of gaseous Ne20 and Ne22 the same?
8.
Is the relation
?,
-
?,
=
R
true only for ideal gases, or is it also true for liquids? If it is not
true for liquids, what formula should be used?
9.
What is the kinetic energy flux in the axial direction for the laminar Poiseuille flow of a New-
tonian liquid in a circular tube?
10.
What is
[P
vl
=
pv
+
[T
vl for Poiseuille flow?
'
See, for example,
R.
J.
Silbey and
R.
A.
Alberty,
Physical
Chemistry,
Wiley, 3rd edition
(2001), s2.11.
