Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

showing a gradual increase of particle thickness, and a decrease in particle defect
density, lattice strain, and compositional variability (Peacor, 1992 ). The evolution of
dioctahedral clay minerals during diagenes is is smectite-random I/S-ordered
I/S-1M
d
illite-2M
1
muscovite.
The transformation of smectite to illite (‘illitization’) occurs when the sediment con-
taining I/S mixed-layer minerals is progressively buried in the basin, and the proportion
of illite layers increases. Potassium ions required for the illitization are supplied mainly
from the alteration of K
+
-feldspar within the sandstones, and from mica within the
shales (Furlan et al., 1996). In this sense the transformation of smectite to illite seems to
occur through a continuous series of interstratified I/S minerals. However, in the
Nankai Trough, Site 808 (an accretionary wedge where the Philippine plate subducts the
Eurasian plate) Masuda et al. (2001) observed that (i) authigenic K
+
-rich smectit e of
high Fe-content forms directly as an alteration product of volcanic glass at a depth of
E500 m below the seafloor with no intermediate precursor; (ii) smectite is largely re-
placed by I/S minerals with Reichweite R ¼ 1 and minor illite and chlorite over a depth
of 500–700 m below the seafloor. Most smectite and I/S minerals are derived from glass
alteration, rather than detrital as usually assumed; and (iii) I/S minerals with discrete
layer sequences (R ¼ 1) and illite co-exist, indicating discontinuities of the transforma-
tion from smectite to illite. This observation is similar to that of Dong et al. (1997),who
observed packets from Gulf Coast and other shales dominated by smectite, I/S minerals
with R ¼ 1(E50% illite), or illite. Thus, the smectite to illite transformation is not
simple and several mechanisms are possible.
On the other hand, S
´
rodon
´
(1999) used the concept of ‘fundamental particles’ to
interpret illitization. This concept was first proposed by McHardy et al. (1982) and
further developed by Nadeau et al. (1984).S
´
rodon
´
suggested that the entities that
evolve during illitization are not mixed-layer crystals but fundamental particles.
The existence of interstratified I/S minerals in the diagenetic environment was
demonstrated by TEM, in apparent opposition to the ‘fundamental particle’ theory.
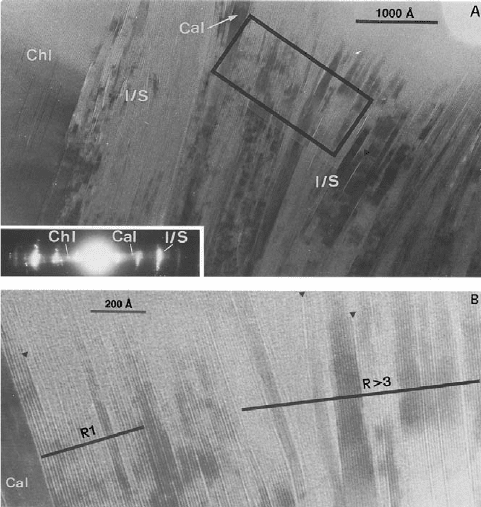
Thus, Nieto et al. (1996) were able to show by TEM the co-existence of chlorite, calcite,
and two I/S mixed-layer minerals (R ¼ 1 with 50% of illite layers and R>3 with more
than 90% illite), both thermodynamically incompatible, over distances of o100 nm
(Fig. 14.4). This assemblage suggests that the evolution of smectite to illite is an Os-
twald-type ripening process (Morse and Casey, 1988) in which phases reach equilib-
rium through progressive steps, with less heterogeneity and closer to the stable phases.
Therefore, two mechanisms of smectite-to-illite transformation are possible (i)
through dissolution- recrystallization without involving mixed-layer minerals (Inoue
and Kitagawa, 1994; Clauer et al., 1997; S
´
rodon
´
, 1999; S
´
rodon
´
et al., 2000) mostly
applicable to bentonite transformation and (ii) through I/S mixed-layer phases
(Eberl and S
´
rodon
´
, 1988; Inoue et al., 1988; Christidis, 1995; Nieto et al., 1996),
applicable to shales and some hydrothermally altered bentonites. Mechanism (ii)
involves solid-state layer-by-layer transformation, including random interstratific-
ation, transition to ordered mixed-layer minerals, and development of long-range
order (Bethke and Altaner, 1986).
14.1. Geological Environments for Clay Formation 1143
Another interesting finding, illustrating the structural and chemical heterogeneity
of these I/S mixed-layer minerals, and their evolution in a basin, was described by
Drits et al. (2002). In oil-source shales from the North Sea (Upper Jurassic), oil
generation takes place simultaneously with diagenetic transformation of I/S. A link
between these two reactions can be the foll owing: NH
3
released during maximum oil
generation is fixed as NH
4
+
cations in the NH
4
+
-bearing mica (tobelite) layers formed
from smectite or vermiculite layers of I/S minerals. This process leads to the for-
mation of four-component illite–tobelite–smectite–vermiculite (I-T-S-V) minerals in
a diagenetic interval, called ‘tobelitization window’. Drits et al. (2002) also found
that the amount of tobelite layers and fixed NH
4
+
increased wi th diagenesis, while
the amount of K
+
fixed in the I/S mixed-layer minerals as well as the proporti on of
K
+
-bearing illite layers remained constant, irrespective of sample location and depth
and degree of diagenetic transformation. They concluded that in these oil-source
shales, diagenesis is accompanied not by smectite illitization, as is generally the case,
but by smectite (or vermiculite) tobelitization. The constant proportion of illite
layers and the constant number of cations are considered as evidence of solid-phase
transformation of the I-T-S-V because any other mechanism would destroy this
Fig. 14.4. (A) TEM image of a sample UG-17 including chlorite, I/S mixed-layer minerals
with R ¼ 1 and R>3, and calcite. (B) lattice fringe image of the area marked in (A) showing
well-defined individual packets of I/S minerals with R ¼ 1 and R>3. From Nieto et al. (1996).
Chapter 14: Genesis of Clay Minerals1144
constancy. Tobelitization of smectite in I/S minerals is probably typical of all oil-
source shales.
Contemporaneous with the smectite-to-illite transformation, significant chlorite is
formed from smectite (through loss of Fe
3+
and Mg
2+
) or kaolinite (by diff usion of
Fe
3+
and Mg
2+
in the structure). The change of trioctahedral smectite to chlorite in
a Mg-rich environment can give a mixed-layer structure of the corrensite type.
During burial diagenetic I/S transformation, Mg
2+
cations can also be fixed in the
smectite layers. The formation of brucite-like sheets can give rise to an I/S-dioc-
tahedral chlorite structure (tosudite) (Lindgreen et al., 2002). Later diagenesis may
lead to neoformation of tosudite and trioctahedral chlorite-berthierine.
Diagenetic changes convert muds into lithified mudrocks, that is, mudstone-
shale-slate. The clay mineral reactions approach chemical equilibrium through
intermediate metastable products. Reactions rates in clays heated by igneous intru-
sion temperatures are many orders of magnitude faster and promote direct trans-
formation of smectite to muscovite without intermediate phases (Merriman, 2002).
Clay mineral reactions require relatively small activation energies because the new
minerals formed have similar compositions and structures to the reactants. As a
result, they can form sequences of reactants and products or reaction series
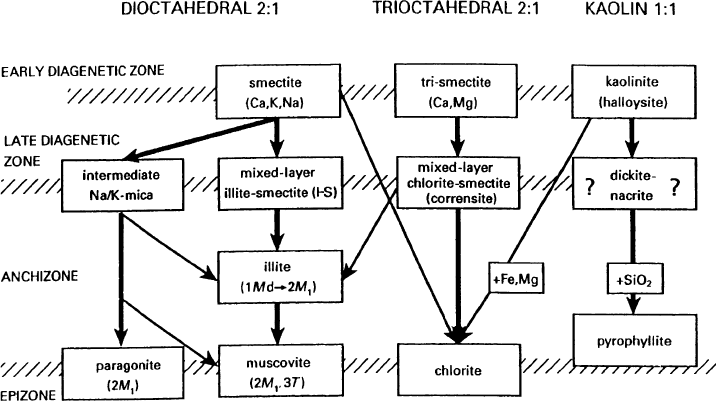
(Merriman and Peacor, 1999). Fig. 14.5 shows the reaction series recognized in
British Lower Paleozoic mudrocks (Merriman and Roberts, 1985, 2001; Fortey,
1989; Roberts et al., 1990, 1991), according to the basic phyllosilicate structure and
Fig. 14.5. Clay mineral reaction series observed in British Lower Paleozoic slate belts. Re-
action proceeds from top to bottom, indicated by heavy arrows. Diagonal arrows indicate
products contributed from one series to another. From Merriman (2002).
14.1. Geological Environments for Clay Formation 1145
increasing diagenetic-metamorphic conditions. For 2:1 dioctahedral clay minerals,
the series includes basically the smectite illitization, and considering the Na/K ratio,
this series evolves to muscovite (K
+
) or paragonite (Na
+
). For the 2:1 trioctahedral
clay minerals (Ca
2+
,Mg
2+
smectites), the smectite chloritization is the fundamental
transformation, usually through corrensite. Finally, for the most simple clay min-
erals, 1:1 dioctahedral ones (kaolinite), the basic transformation is the formation of
more ordered polytypes (mainly dickite), and the possibility of pyrophyllite forma-
tion in the anchizone–epizone conditions. These series described for the British
Lower Paleozo ic were also recognized in other belts, that is, in the Cantabrian
Cordillera (Spain) (Gala
´
n and Aparicio, 1980 ).
Clay mineral assemblages found in slates can also be related to the geotectonic
setting and thermal history of the basin (Merriman, 2002). Extensional basins are
characterized by high-heat flow (>35 1C/km) and hydrothermal activity, an d tend to
have a greater diversity of transformed clay minerals containing both K
+
- and Na
+
-
rich products of the 2:1 dioctahedral reaction series. Pyrophyllite, rectorite, corren-
site, and paragonite can be recognized but kaolinite is rare. In contrast, clay
assemblages that evolve in convergent low-heat flow basins generally contain fewer
mineral species, simply K
+
-white mica and chlorite, and Na
+
-mica and pyrophyllite
are rare or absent. In extensional basins Na
+
ions may come from low-temperature
mixing of hydrothermal fluids and sea water. Such fluids are unavailable in con-
vergent basins because of a lack of volcanic activity.
Over the last few decades, clay mineral assemblages and some parameters, such as
the Ku
¨
bler index for illite, smectite–il lite reactions, chlorite composition, and the
Arkay index for chlorite, were used as geothermometers and geobarometers for
assessing the conditions in basins that were submitted to burial diagenesis and very
low-grade metamorphism. However, as Essene and Peacor (1995) pointed out, the
use of such systems and parameters does not provide accurate geother mometers
because most of them are not based on equilibrium reactions.
Recent research demonstrated that most clay minerals are out of equilibrium with
their environment. Clay mineral transformations are equivalent to Ostwald-ripening
steps, driven by the potential for minimum free energy, and clay minerals may reach
equilibrium only in very low or low-grade metamorphism. The application of clay
mineral assemblages is based on physical calibration as observed for natural clay-bearing
systems. The repeatability of clay mineral assemblages in space and time, as a function of
increasing P–T conditions, is often assumed to imply equilibrium. However, this ob-
servation is not evidence for equilibrium although it is a necessary condition (Essene and
Peacor, 1995). Most clay mineral associations are metastable, and the chemical reaction
kinetics depend on a great variety of parameters and circumstances, such as time, fluid/
rock ratio, tectonic history (deformation), starting material, pressure, and temperature.
On the other hand, ‘retrograde’ reactions can occur where I/S mixed-layer min-
erals form by replacement of metamorphic illite (Jiang et al., 1990), smectite derives
from chlorite (Nieto et al., 1994), or smectite and highly expandable I/S minerals
from illite (Zhao et al., 1999).
Chapter 14: Genesis of Clay Minerals1146
Another interesting formation of clay minerals in this environment is due to the
devitrification of volcanic ash, leading to the formation of massive smectite (bento-
nite). The process seems to consist of slow ionic diffusion caused by a slow burial
rate, and hence a low compaction rate in an open chemical system. In many bento-
nite beds I/S mixed-layer minerals with high K
+
content are found in contact with
the enclosing sed imentary rock, indicating that the diffusion process gradually
transforms smectite into I/S mixed-layer minerals over a long period of time.
A special case of great practical interest is the formation by diagenesis of clay
minerals in sandstone pores. Connected pores in sandstone create permeability.
Since sandstones can serve as possible hydrocarbon reservoirs, porosity and perme-
ability are two important factors. Both parameters can decreas e due to the diagenetic
formation and growth of clays in pores and in the passages connecting them, hin-
dering the passage of fluids and reducing the economic value of the reservoir. Ka-
olinite is the most frequent clay mineral formed, often as vermicular ‘books’ of
stacked layers but I/S interstratified clay minerals, berthierine, and lath-shaped illite
are also common.
Diagenetic kaolinite can form by flushing sandstones with meteoric water. Ka-
olinite-feldspar assemblages are stable until 120–140 1C and react to form illite.
Huang et al. (1986) confirmed the role of fluid/rock ratio in altering feldspars into
kaolinite or illite. Ehrenberg et al. (1993) and Ruiz Cruz and Andreo (1996) doc-
umented the kaolinite-dickite transition at E120 1C.
According to Beaufort et al. (1998) the kaolinite-to-dickite reaction proceeds by
gradual structural changes concomitant with crystal coarsening and a ch ange from
booklet to blocky morphology (Fig. 14.6). The crystallization of dickite follows two
different pathways: (i) accretion of new material from the dissolution of unstable
kaolinite particles and/or detrital minerals, or coarser metastable kaolinite and (ii)
neoformation of ordered dickite by dissolution–crystallization process. They suggest
that the kaolinite-to-dickite reaction has a petrogenetic significance, useful for re-
constructing the burial diagenesis of sedimentary basins, and for petroleum and gas
exploration.
Lanson et al. (2002) reviewed the authigenic minerals formed in sandstones during
burial diagenesis. While early precipitation of kaolinite is generally related to flush-
ing by meteoric waters and as a consequence of feldspar dissolution in an open
system, subsequent diagenetic kaolinite-to-dickite transformation probably results
from invasion by acidic fluids of organic origin. This conversion is kinetically con-
trolled and dickite is the most stable polytype in sandstone. However, dickite is not
formed exclusively by diagenetic transformation of kaolinite. It can also form from
dissolution of K
+
-feldspars and other Al-rich sil icates with increasing temperature,
probably in the presence of organic acids. On the other hand, illite crystallization at
the expense of kaolinite implies that an energy barrier is overcome either by an
increase in K
+
/H
+
activity ratio in solution or by a temperature increase. Illitization
of kaolinite is not necessa rily coupled to dissolution of K-feldspars as usually de-
scribed, but a potassium source is required.
14.1. Geological Environments for Clay Formation 1147
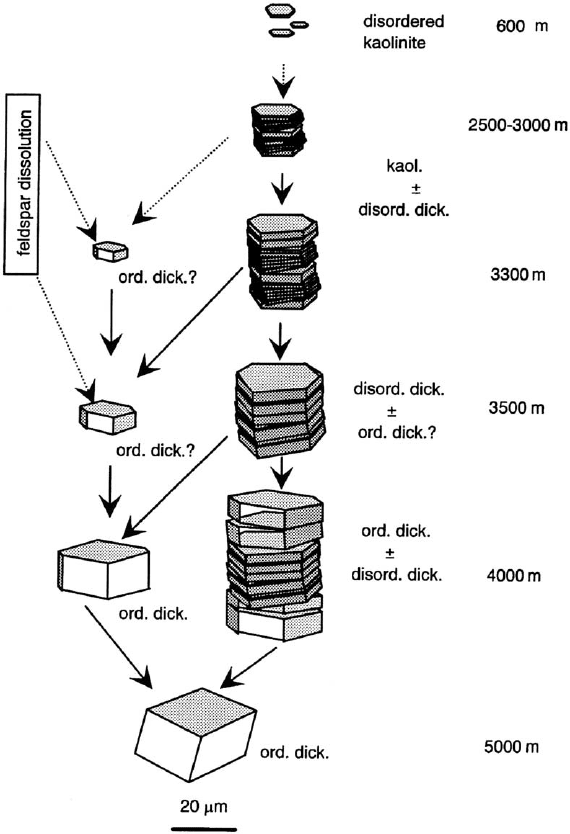
Fig. 14.6. Schematic model of the kaolinite-to-dickite reaction involving both morphological
and structural changes as a result of water–rock interaction for increasing burial depths in
sandstone reservoirs. Arrows indicate the transfer of matter due to material redistribution
involved in the dissolution–crystallization processes. After Beaufort et al. (1998).
Chapter 14: Genesis of Clay Minerals1148
In summary, we can say that early burial diagenesis and the passage to low-grade
metamorphism (anchimetamorphism) transform clay minerals into other minerals
until environmental equilibrium is reached. Typical clay minerals formed during
diagenesis are the I/S interstratified minerals, which are intermediate phases in
smectite illitization. This transformation was studied in much detail, because the
reaction can indu ce petroleum migration and build up geopressures by liberating
water from smectite interlayers. Soluble SiO
2
,Fe
3+
, and Mg
2+
liberated during
illitization may cause cementation in the pores of sedim entary rocks (see Altaner and
Bethke (1988) and references therein). With increasing burial other phyllosilicates
can be formed, such as pyrophyllite and paragonite. These minerals are in metastable
equilibrium with I/S mixed-layer minerals in the anchimetamorphism zone. Chlorite
can also be formed from smectite or kaolinite. The first stage of transformation can
give rise to corrensite. On the other hand, massive smectite can be formed from the
devitrification of volcanic ash.
Kaolinite is a typical clay mineral formed in sandstone pores by direct preci-
pitation. At 120–140 1C it can transform into illite (by reaction with feldspars) or
dickite. Dickite can also form directly from dissolution of feldspars (Lanson et al.,
2002).
XRD was fundamental to following the evolution of clay minerals in a basin as a
function of depth, time, and tectonics. The application of other instrumental tech-
niques, notably HRTE M, provided much valuable information, and novel insights
into clay mineral formation and transformation. More studies are still necessary, in
particular, on the relationship between these process es and their geotectonic setting
(Merriman, 2002).
14.1.4. Hydrothermal Alteration
As mentioned before, intrusion of plutonic rocks and the deposition of volcanic
events lead to wall-rock alteration and transformation of some rock-forming min-
erals into clayey materials. Clays can be genetically associated with late-stage de-
posits (chloritization), pegmatites, albitite-greissen deposits (musco vitization,
chloritization), and hydrothermal veins. The veins are most important because crys-
tal-chemical variations of clay minerals are particularly related to the fluid compo-
sition and controlled by the type of altered rock.
Hydrothermal alteration involves water–rock interaction at temperature above
E50 1C. In general, the altered material forms at higher temperatures than those of
the hydrothermal fluids, hence the minerals are unstable in the presence of water at
the temperatures considered. The fluids are aqueous and contain gaseous compo-
nents and dissolved materials.
Hydrothermal systems are open to components where convecting fluids produce
maximum water/rock ratios, and depths may be shallow (Henley, 1985). Very
different clay minerals may form at the same time, but at different temperatures. At
300–400 1C the clay alteration facies include sericite or mica, potassium feldspars and
14.1. Geological Environments for Clay Formation 1149
chlorite, very simila r to that of the greissen deposits. As temperature decreases illite
and kaolinite predominate with some I/S mixe d-layer minerals. At still lower tem-
peratures smectite or kaolinite can be the most important clay minerals. The most
striking examples of hydrothermal alteration are the production of pure kaolin and
silica. These minerals can occur on the scale of kilometers, and are of economical
interest.
In epithermal ore deposits, found in the upper (o1–2 km) geothermal systems
where predominantly meteoric fluids convect, interaction with the host rock takes
place at temperatures of 200–300 1C. In general, these systems are relatively short-
lived, remaining active over periods of hundreds of thousands of years or less (Henley
and Ellis, 1983). Nonetheless, interesting clay (e.g., kaolin) deposits can be produced.
The following three cases illustrate the diversity of clay minerals formed in this
environment under the influence of altered rock type, P and T, and fluid chemistry.
In the Golden Cross epithermal Au–Ag deposit at Waihi, New Zealand hosted by
andesitic and dacitic lavas, hydrothermal alteration of the rocks gives rise to quartz,
adularia, calcite, clay minerals (chlorite, illite, kaolinite, I/S mixed-layer minerals,
and smectite), and others. Accor ding to Tillick et al. (2001) all phyllosilicates are
neoformed directly from the fluid s. I/S mixed-layer minerals (R ¼ 1) and micas form
simultaneously without intermediate series, as Masuda et al. (2001) observed for the
burial diagenes is of mudsto nes. The illite content in the I/S minerals increases with
depth and proximity to the central vein system. Smectites co-exist with (R ¼ 1),
rather than (R ¼ 0), I/S minerals.
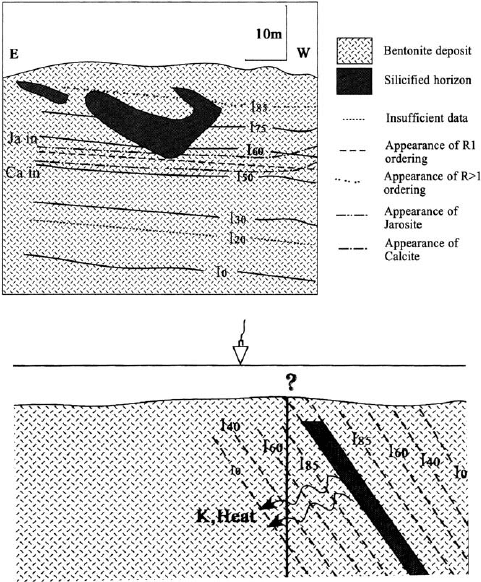
The second case is the hydrothermal alteration of ben tonites of the Tsantili de-
posit in Milos Island, Greece. The original Wyoming-type montmorillonite was il-
litized along a 40 m vertical profile, forming I/S mixed-layer minerals with different
proportions of expandable layers and different ordering (Fig. 14.7). The alteration is
characterized by a massive incorporation of K
+
and removal of Si
4+
,Na
+
,Ca
2+
,
Mg
2+
,andFe
3+
from the rock. The hydrothermal alteration is probably associated
with the emplacement close to the parent bentonite of barite veins at o200 1C.
Illitization is controlled by temperature, K
+
availability, and fluid/rock ratio. It can
be considered to be the result of K
+
-metasomatism. As the potassium is derive d
from the dissolution of authigenic K
+
-feldspar, the I/S mineral particles are appar-
ently formed by solid-state transformation. According to Christidis (1995), the ill-
itization and spatial distribution of expandable miner als could be used as a guide for
the exploration of mineral deposits.
The third case concerns the intrusion of a magmatic rock in a sedimentary series,
leading to contact metamorphism in the host rock. Subsequent hydrothermal alter-
ation usually yields clayey materials. For instance, in the Priego de Co
´
rdoba area
(Subbetic zone of the Betic Cordilleras, Spain), a lacolith of stratiform dolerite
intruded marly sediments at quite shallow depths below the ocean floor during the
intracontinental rifting phase. In the first stage, contact metamorphism caused crys-
tallization of Ca
2+
-silicates at about 500 1C. During a process of hydrother mal
alteration superimposed on the contact aureole, chlorite was formed in the area
Chapter 14: Genesis of Clay Minerals1150
closest to the volcanic rocks in a first step. In the zone farthest from the contact area
corrensite was formed, but a later cooling phase led to crystallization of saponite in
the host rock, and di- and trioctahedral smectites inside the volcanic rock (Abad
et al., 2003 ). Temperature can decrease from E300 1C (chlorite) to 270–200 1C
(chlorite–smectite interstratifications) in the prograde phase, and to 180–130 1C
(saponite) in the retrograde phase (Stakes and O’Neil, 1982; Dudoignon et al., 1997).
Deep-sea hydrothermal alteration is rather different from the alteration described
above. Hydrothermal alteration in basic and acidic rocks tends to produce Mg-and
Fe-free clay mineral assemblages, whereas alteration of deep-sea basalts at very low
temperatures yields Fe-rich clays. With decreasing temperatures from E300 1C the
A)
B)
Fig. 14.7. (A) Schematic cross-section of the south sector of the Tsantili bentonite deposit
(Greece). Ii (i ¼ 0 85) is the illite content of the I/S mixed-layer minerals. Ca in ¼ presence
of calcite incompatible with jarosite; Ja in ¼ presence of jarosite incompatible with calcite; (B)
Simplified model proposed for the illitization of Wyoming-type montmorillonite in the Tsantili
deposit in relation to barite vein (vertical line represents the cross-section of (A)). K,
Heat ¼ K
+
availability and temperature are decreasing from the deposit. After Christidis
(1995).
14.1. Geological Environments for Clay Formation 1151
sequence of clay minerals in the altered basalts is serpentine-saponit e -celado-
nite-nontronite. The last mineral is found in the ‘weathering’ zone on basalts, that
is, at the sea water–rock interface.
In summary, clay minerals formed by hydrothermal activity are not related to
sedimentary layers or weathering crusts. In contrast with the other environm ent
described, zonation from the heat source is a characteristic of this complex envi-
ronment, which can be very variable over short distances. Clay minerals formed can
have an economic interest, and a detailed study can provide information about their
use as indicators for exploring ore deposits.
14.2. ORIGIN OF CLAY DEPOSITS OF ECONOMIC INTEREST
Commercial clays, that is, clays used as raw material in industry rank as the
leading industrial rocks in both tonnages and total value. Clay is a rock historically
linked to humans in their agricultural, industrial, and cultural development. It is a
relatively abundant raw material with many different applications, encompass ing a
wide spectrum of products. These range from low-value materials, produced with a
minimal degree of processing (e.g., certain plastic clays for bricks) to high added-
value materials (e.g., refined kaolins for paper coating) (see Chapter 10.1). About
90% of production comprises building clays, or miscellaneous clays for structural
products. Only 10% of deposits are largely composed of a clay mineral (kaolin,
bentonite, fuller’s earths, etc.); these are generally referred to as ‘special clays’.
The origin of commercial clays is of great basic interest because the uncommonly
massive concentrations of very pure clay material are produced under special con-
ditions. Geological criteria shown by genetic studies can be used for the exploration
and mining of new deposits.
Here we give a brief overview of the genesis of the principal clay deposits, other
than structural clays. The latter are sedimentary formations (marine and continental)
abundantly distributed throughout the world from the Carboniferous to the Cenozoic.
14.2.1. Kaolins
Kaolinite can be formed by weathering (residual kaolins) and hydrothermal activity
(hydrothermal kaolin), or occur as an authigenic sedimentary mineral. Residual and
hydrothermal kaolins, called primary kaolins, are formed in situ by surface and
underground waters or hydrothermal fluids. Sedimentary kaolins, called secondary
kaolins, are composed of kaolinized material from a source area that was eroded,
transported, and deposited in a continental or coastal environment. Most kaolinites
in sedimentary kaolins are formed before deposition, in a primary environment, but
some can be authigenic after the deposition of the arkosic or sandy material, usually
by the action of underground water.
Chapter 14: Genesis of Clay Minerals1152
